Question: solve questions a b and c solve question 2 complete subquestions a, b and c solve them Q2) A) Answer any three of the following:
solve questions a b and c
solve question 2 complete subquestions a, b and c solve them
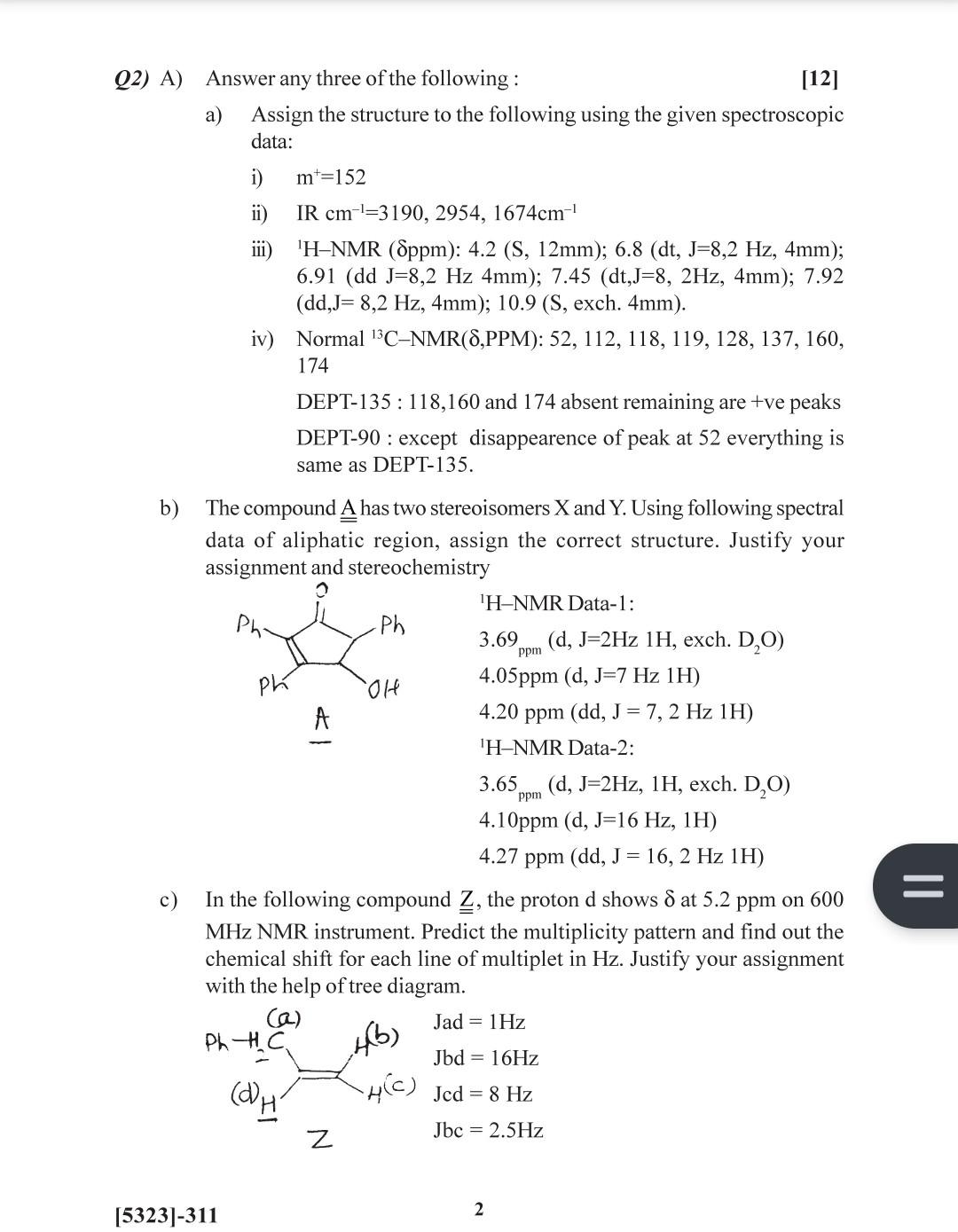
Q2) A) Answer any three of the following: [12] a) Assign the structure to the following using the given spectroscopic data: i) m+=152 ii) IR cm1=3190,2954,1674cm1 iii) 1HNMR(ppm):4.2(S,12mm);6.8(dt,J=8,2Hz,4mm); 6.91(ddJ=8,2Hz4mm);7.45(dt,J=8,2Hz,4mm);7.92 (dd,J=8,2Hz,4mm);10.9 (S, exch. 4mm). iv) Normal 13CNMR(,PPM):52,112,118,119,128,137,160, 174 DEPT-135: 118,160 and 174 absent remaining are +ve peaks DEPT-90 : except disappearence of peak at 52 everything is same as DEPT-135. b) The compound A has two stereoisomers X and Y. Using following spectral data of aliphatic region, assign the correct structure. Justify your assignment and stereochemistry 'H-NMR Data-1: 3.69ppm(d,J=2Hz1H, exch. D2O) 4.05ppm(d,J=7Hz1H) 4.20ppm(dd,J=7,2Hz1H) 'H-NMR Data-2: 3.65ppm(d,J=2Hz,1H, exch. D2O) 4.10ppm(d,J=16Hz,1H) 4.27ppm(dd,J=16,2Hz1H) c) In the following compound Z, the proton d shows at 5.2ppm on 600 MHz NMR instrument. Predict the multiplicity pattern and find out the chemical shift for each line of multiplet in Hz. Justify your assignment with the help of tree diagram. [5323]-311 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


