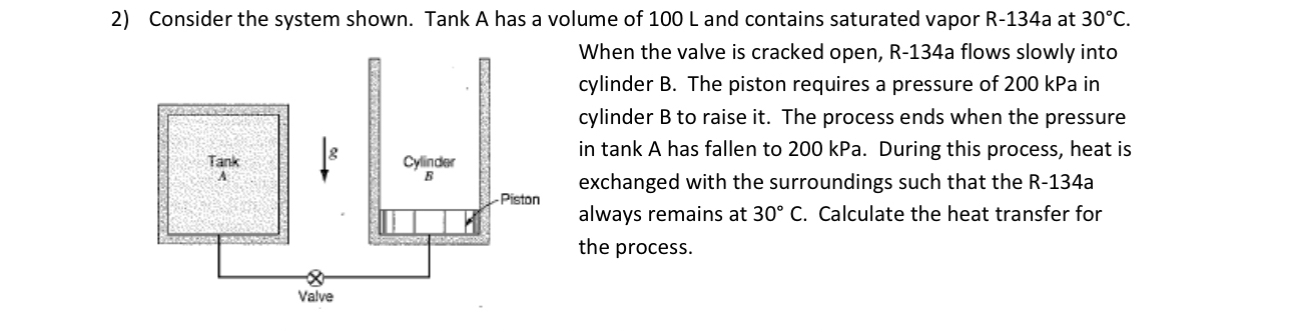
Question: SOLVE STEP BY STEP.Consider the system shown. Tank A has a volume of 1 0 0 L and contains saturated vapor R - 1 3
SOLVE STEP BY STEP.Consider the system shown. Tank A has a volume of L and contains saturated vapor at When the valve is cracked open, R a flows slowly into cylinder B The piston requires a pressure of kPa in cylinder B to raise it The process ends when the pressure in tank A has fallen to kPa During this process, heat is exchanged with the surroundings such that the Ra always remains at Calculate the heat transfer for the process.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


