Question: Solve the following problem by the Hydrated Bases method and the formula. A crystallizer-evaporator is fed with 80,000 lb/h of an aqueous solution containing 25%
Solve the following problem by the Hydrated Bases method and the formula.
A crystallizer-evaporator is fed with 80,000 lb/h of an aqueous solution containing 25% by weight of MgSO4 at 200F. The solution is cooled to 130F and evaporates 30,000 lb/h of water. Calculate:
1. The % of MgSO4 recovered.
2. Crystal production
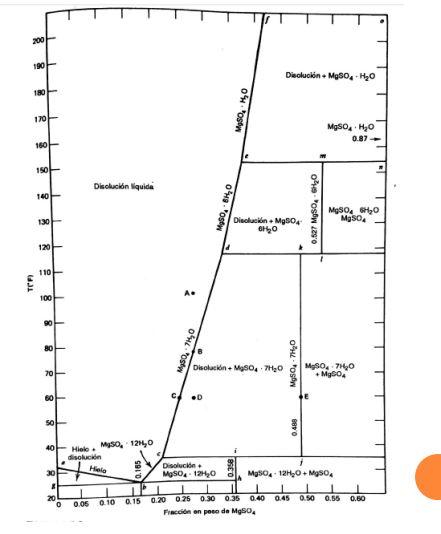
200 100 Diectuokin + MgSO-440 180 Mg50,0 170 MgSOHO 0.87 160 150 Disolucin liquida 140 O'Hosen 0.527 MgSO4 20 MgSO4 SHO MgSO4 130 Disolucin Mg504 GHO 120 110 TER A. 100 90 80 Mon: 740 Disclackin+MSO7H0 O'Hostw 70 MgSO47140 + Myoo 60 D 50 0488 40 30 Hielo MySO 1214,0 disolucin Hiero Disolucin 1 MgSO4. 12,0 MgSO4.12H2O + MgSO4 1 I 1 0.05 010 0.15 0.20 0.250.300.35 0.40 0.45 0.50 0.55 Freccin en pose de Mg04 1 0.50 20 0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


