Question: Solve the Hr for the following balanced equation using Hess's Law and the given data [ begin{array}{ll} multicolumn{1}{c}{mathrm{C}_{3} mathrm{H}_{8(mathrm{~g})}+5 mathrm{O}_{2(mathrm{~g})} ightarrow 3 mathrm{CO}_{2(mathrm{~g})}+mathbf{4 mathrm {
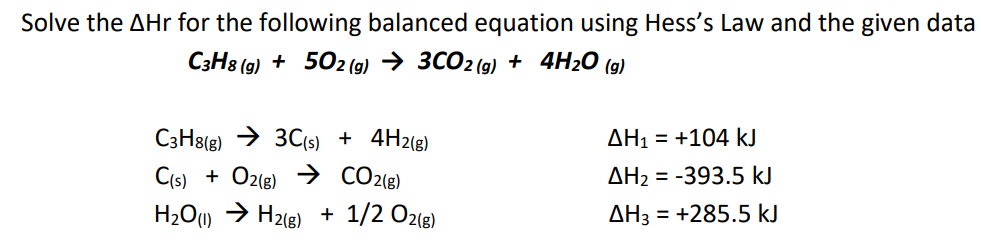
Solve the Hr for the following balanced equation using Hess's Law and the given data \[ \begin{array}{ll} \multicolumn{1}{c}{\mathrm{C}_{3} \mathrm{H}_{8(\mathrm{~g})}+5 \mathrm{O}_{2(\mathrm{~g})} ightarrow 3 \mathrm{CO}_{2(\mathrm{~g})}+\mathbf{4 \mathrm { H } _ { 2 } \mathrm { O }}(\mathrm{g})} \\ \mathrm{C}_{3} \mathrm{H}_{8(\mathrm{~g})} ightarrow 3 \mathrm{C}_{(\mathrm{s})}+4 \mathrm{H}_{2(\mathrm{~g})} & \Delta \mathrm{H}_{1}=+104 \mathrm{~kJ} \\ \mathrm{C}_{(\mathrm{s})}+\mathrm{O}_{2(\mathrm{~g})} ightarrow \mathrm{CO}_{2(\mathrm{~g})} & \Delta \mathrm{H}_{2}=-393.5 \mathrm{~kJ} \\ \mathrm{H}_{2} \mathrm{O}_{(\mathrm{l})} ightarrow \mathrm{H}_{2(\mathrm{~g})}+1 / 2 \mathrm{O}_{2(\mathrm{~g})} & \Delta \mathrm{H}_{3}=+285.5 \mathrm{~kJ} \end{array} \]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


