Question: Solve the problem using the 4th Order Runge-Kutta method. Do it in EXCEL and MATLAB. The elementary reactions below take place in a constant volume
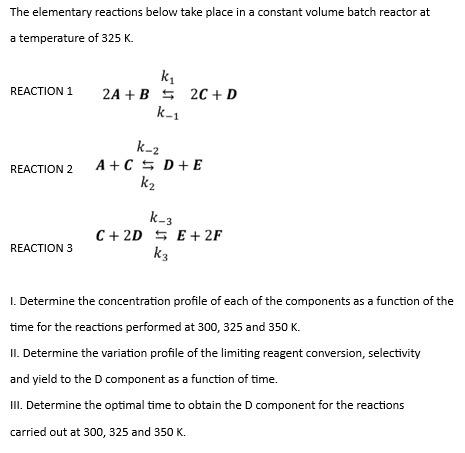

The elementary reactions below take place in a constant volume batch reactor at a temperature of 325K. REACTION 1 2A+Bk1k12C+DA+Ck2D+Ek2C+2Dk3k3S+2F REACTION 3 I. Determine the concentration profile of each of the components as a function of the time for the reactions performed at 300,325 and 350K. II. Determine the variation profile of the limiting reagent conversion, selectivity and yield to the D component as a function of time. III. Determine the optimal time to obtain the D component for the reactions carried out at 300,325 and 350K. Initial concentration of ACAO=5.0mol/L Initial concentration of BCBD=3.0mol/L Initial concentration of CCCO=0.0mol/L Initial concentration of DCDO=0.0mol/L Initial concentration of EECD=0.0mol/L Initial concentration of FCFD=0.0mol/L Reactor Volume V0=5.0L Rate constant of reaction 1 at 325Kk1,325K=0.001L3mol2min1 Rate constant of reaction 2 at 325Kk2,325K=0.002L3mol2min1 Rate constant of reaction 3 at 325Kk3,325K=0.003L3mol2min1 Reaction activation energy 1EA1=20000Jmol1 Activation energy of reaction 2EA2=30000Jmol1 Activation energy of reaction 3EA3=60000Jmol1 Reaction equilibrium constant 1 at 325KK1,325K=50.00 Equilibrium constant of reaction 2 at 325KK2,325K=10.00 Reaction equilibrium constant 3 at 325KK3,325K=60.00 Reaction enthalpy 1Hr1=10000Jmol1 Reaction enthalpy 2Hr2=30000Jmol1 Reaction enthalpy 3Hr3=20000Jmol1 Gas constant R=8.314Kmol1K1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


