Question: solve the question below and draw the graph in detail please In the dilute concentration region equilibrium data for SO2 distributed between air and water
solve the question below and draw the graph in detail please 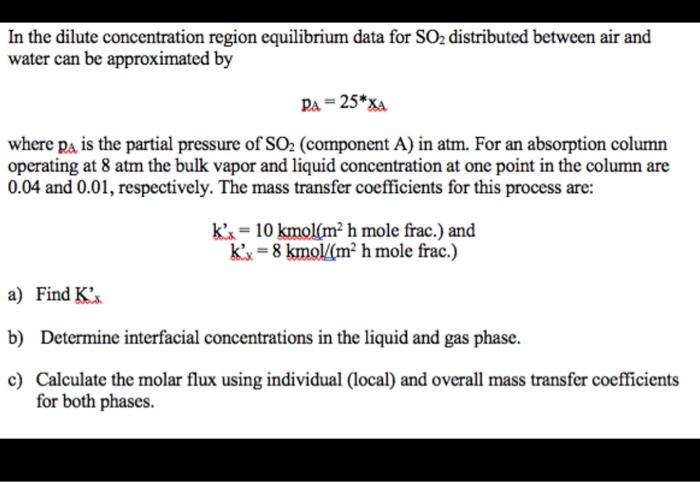

In the dilute concentration region equilibrium data for SO2 distributed between air and water can be approximated by PA = 25*XA where pa is the partial pressure of SO2 (component A) in atm. For an absorption column operating at 8 atm the bulk vapor and liquid concentration at one point in the column are 0.04 and 0.01, respectively. The mass transfer coefficients for this process are: kx= 10 kmol(m? h mole frac.) and kx = 8 kmol/(m h mole frac.) a) Find K's b) Determine interfacial concentrations in the liquid and gas phase. c) Calculate the molar flux using individual (local) and overall mass transfer coefficients for both phases
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


