Question: Solve the questions and include excel Problem 2 (20 points): An equimolar ternary mixture of acetone, n-butane, and ammonia at 1 MPa is to be
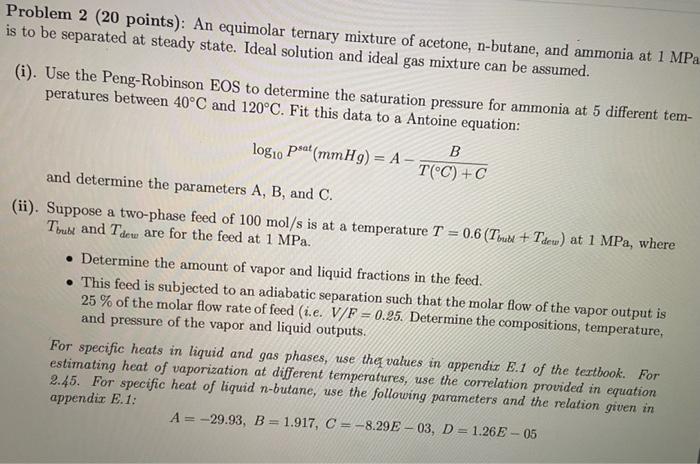
Problem 2 (20 points): An equimolar ternary mixture of acetone, n-butane, and ammonia at 1 MPa is to be separated at steady state. Ideal solution and ideal gas mixture can be assumed. (i). Use the Peng-Robinson EOS to determine the saturation pressure for ammonia at 5 different tem- peratures between 40C and 120. Fit this data to a Antoine equation: B logio Pat (mmHg) = A - T(C) +C and determine the parameters A, B, and C. (ii). Suppose a two-phase feed of 100 mol/s is at a temperature = 0.6 (Tun +Tdew) at 1 MPa, where Toube and Tdou are for the feed at 1 MPa. Determine the amount of vapor and liquid fractions in the feed. . This feed is subjected to an adiabatic separation such that the molar flow of the vapor output is 25 % of the molar flow rate of feed (i.e. V/F = 0.25. Determine the compositions, temperature, and pressure of the vapor and liquid outputs. For specific heats in liquid and gas phases, use the values in appendix E.1 of the textbook. For estimating heat of vaporization at different temperatures, use the correlation provided in equation 2.45. For specific heat of liquid n-butane, use the following parameters and the relation given in appendix E.1: A = -29.93, B = 1.917, C = -8.29E - 03, D = 1.26E - 05
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


