Question: solve these questions please HOMEWORK (2) PLEASE WRITE YOUR ANSWERS IN THE YELLOW HIGHLITED SPACE: 1) A 5.0 gram of CHC. (78g/mol) is burned in
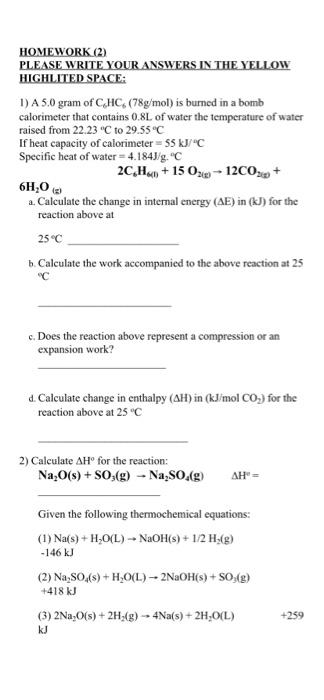
HOMEWORK (2) PLEASE WRITE YOUR ANSWERS IN THE YELLOW HIGHLITED SPACE: 1) A 5.0 gram of CHC. (78g/mol) is burned in a bomb calorimeter that contains 0.8L of water the temperature of water raised from 22.23 C to 29.55C If heat capacity of calorimeter = 55 kJ/C Specific heat of water = 4.1843.g. "C 2C,H,0 +15 026-12CO3 + 6H,0 2. Calculate the change in internal energy (AE) in (kJ) for the reaction above at 25C b Calculate the work accompanied to the above reaction at 25 "C c. Does the reaction above represent a compression or an expansion work? d. Calculate change in enthalpy (AH) in (kJ/mol CO.,) for the reaction above at 25 C 2) Calculate H for the reaction: Na O(s) + SO:(g) - Na:SO ( " = Given the following thermochemical equations: (1)Na(s) + H20(L) - NaOH(s) + 1/2 H (8) -146 kJ (2) Na2SO4(s) +H20(L) - 2NaOH(s) + SO42) +418 kJ (3) 2Na,C(s) + 2H45) -- Na(s) + 2H,0(L) +259 kJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


