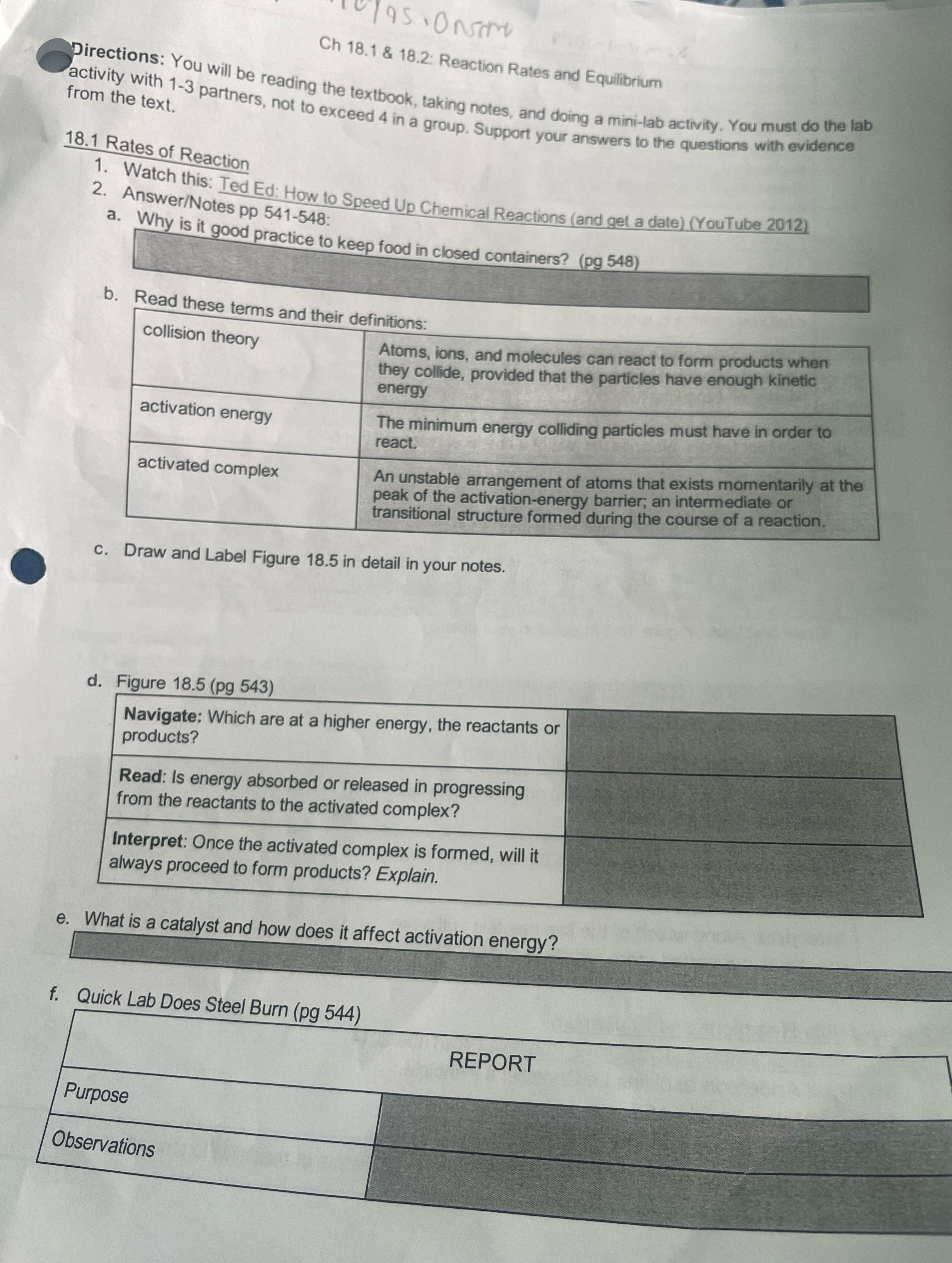
Question: solve this 10795 . Onsome Ch 18.1 & 18.2: Reaction Rates and Equilibrium Directions: You will be reading the textbook, taking notes, and doing a
solve this
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


