Question: Solve this question completely.All the data is mentioned u need to give full solution and it must be accurate.I will rate u 1. Using the
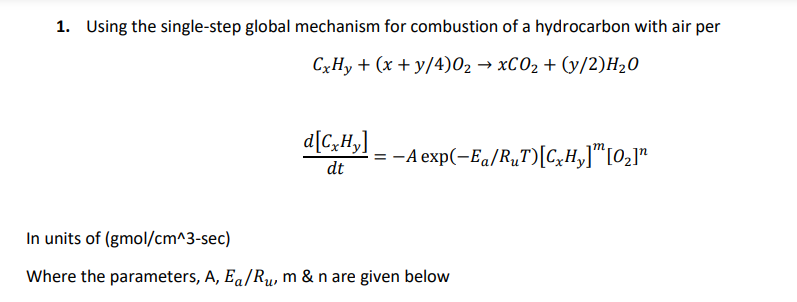
 Solve this question completely.All the data is mentioned u need to give full solution and it must be accurate.I will rate u
Solve this question completely.All the data is mentioned u need to give full solution and it must be accurate.I will rate u
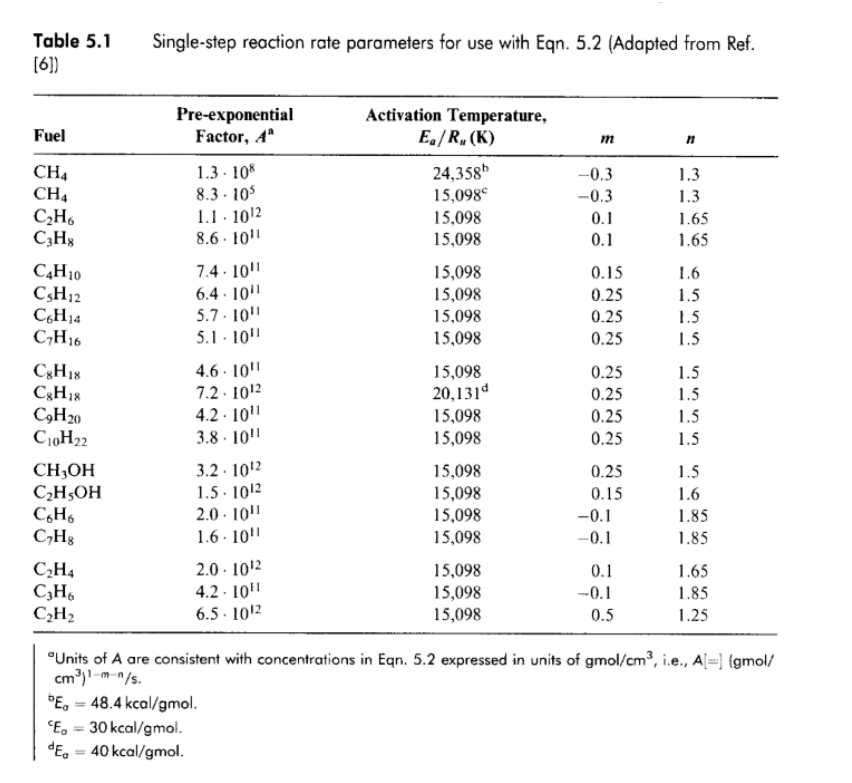
1. Using the single-step global mechanism for combustion of a hydrocarbon with air per CxHy + (x + y/4)02 XCO2 + (y/2)H20 d[C,H,] dt --A exp(-Eq/R,T)[C_H,][02] In units of (gmol/cm^3-sec) Where the parameters, A, Ea/Rw, m & n are given below Table 5.1 [6]) Single-step reaction rate parameters for use with Eqn. 5.2 (Adapted from Ref. Pre-exponential Factor, A Activation Temperature, E/R,(K) Fuel m n CH, CH4 C2H CzH C4H10 CsH2 CH 1.3 1.3 1.65 1.65 1.3. 108 8.3. 105 1.1. 1012 8.6. 101 7.4. 10" 6.4.2011 5.7. 10" 5.1. 1011 24,3585 15,098 15,098 15,098 15,098 15,098 15,098 15,098 -0.3 -0.3 0.1 0.1 0.15 0.25 0.25 0.25 16 1.5 1.5 1.5 C7H16 CH CgH C9H20 C10H22 , C2H5OH C6H CH C2H4 CzH C2H2 4.6. 10" 7.2. 1012 4.2.1011 3.8. 1011 3.2. 1012 1.5. 1012 2.0. 1011 1.6. 101 2.0. 1012 4.2.1011 6.5. 1012 15,098 20,1314 15,098 15,098 15,098 15,098 15,098 15,098 0.25 0.25 0.25 0.25 0.25 0.15 -0.1 -0.1 1.5 1.5 1.5 1.5 1.5 1.6 1.85 1.85 15,098 15,098 15,098 0.1 -0.1 0.5 1.65 1.85 1.25 Units of A are consistent with concentrations in Egn. 5.2 expressed in units of gmol/cm3, i.e., A[=) (gmol/ cm).m-/s. E = 48.4 kcal/gmol. E = 30 kcal/gmol. de = 40 kcal/gmol. Compare the rates of fuel carbon conversion of CO2 for and equivalence ratio of =1 at p = 1 atm, T = 1600 K for the following fuels (make certain to include nitrogen form the air in determining your products' concentrations) a. Methane, CH4 b. Propane, C3H8 Octane, C8H18 C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


