Question: solve this . Unsaturated fatty acids have double bonds between the carbons (C=C) and are have fewer hydrogen atoms because of these double bonds. Unsaturated
solve this
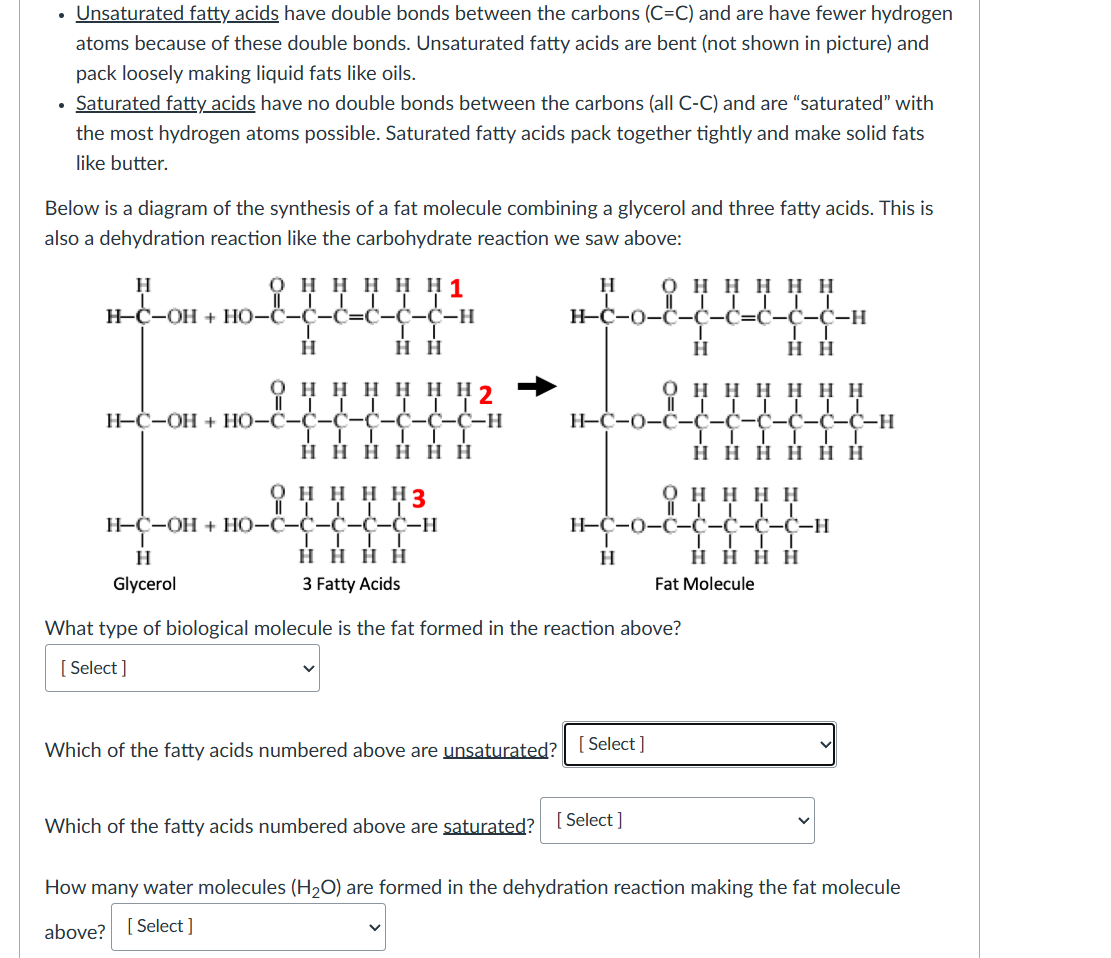 . Unsaturated fatty acids have double bonds between the carbons (C=C) and are have fewer hydrogen atoms because of these double bonds. Unsaturated fatty acids are bent (not shown in picture) and pack loosely making liquid fats like oils. . Saturated fatty acids have no double bonds between the carbons (all C-C) and are "saturated" with the most hydrogen atoms possible. Saturated fatty acids pack together tightly and make solid fats like butter. Below is a diagram of the synthesis of a fat molecule combining a glycerol and three fatty acids. This is also a dehydration reaction like the carbohydrate reaction we saw above: H H H H H H H-C-OH + HO- HHHHHH HHHHHH HH HH3 H-C-OH + HO-c-c-- OHHHH H-C-O-2-C-C-C-H H HHHH H HHHH Glycerol 3 Fatty Acids Fat Molecule What type of biological molecule is the fat formed in the reaction above? [ Select ] Which of the fatty acids numbered above are unsaturated? [ Select ] Which of the fatty acids numbered above are saturated? [ Select ] How many water molecules (H20) are formed in the dehydration reaction making the fat molecule above? [ Select ]
. Unsaturated fatty acids have double bonds between the carbons (C=C) and are have fewer hydrogen atoms because of these double bonds. Unsaturated fatty acids are bent (not shown in picture) and pack loosely making liquid fats like oils. . Saturated fatty acids have no double bonds between the carbons (all C-C) and are "saturated" with the most hydrogen atoms possible. Saturated fatty acids pack together tightly and make solid fats like butter. Below is a diagram of the synthesis of a fat molecule combining a glycerol and three fatty acids. This is also a dehydration reaction like the carbohydrate reaction we saw above: H H H H H H H-C-OH + HO- HHHHHH HHHHHH HH HH3 H-C-OH + HO-c-c-- OHHHH H-C-O-2-C-C-C-H H HHHH H HHHH Glycerol 3 Fatty Acids Fat Molecule What type of biological molecule is the fat formed in the reaction above? [ Select ] Which of the fatty acids numbered above are unsaturated? [ Select ] Which of the fatty acids numbered above are saturated? [ Select ] How many water molecules (H20) are formed in the dehydration reaction making the fat molecule above? [ Select ]
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


