Question: solve using ice table A student uses a solution of 0.30mol/LHCl to titrate a 45.0mL solution of 0.35mol/L methylamine, CH3,NH2. Calculate the pH at this
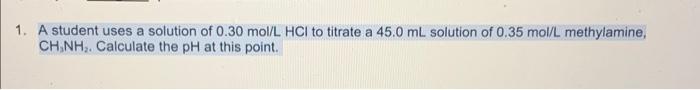
A student uses a solution of 0.30mol/LHCl to titrate a 45.0mL solution of 0.35mol/L methylamine, CH3,NH2. Calculate the pH at this point
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


