Question: Solve using matrices or linear algebra (1 point) Consider the chemical reaction aP4O10+bCaF2cPF5+dCa3(PO4)2, where a,b,c, and d are unknown positive integers. The reaction mush be
Solve using matrices or linear algebra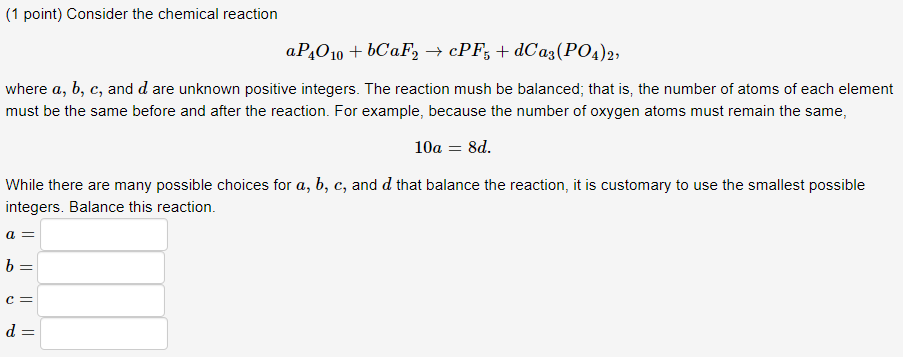
(1 point) Consider the chemical reaction aP4O10+bCaF2cPF5+dCa3(PO4)2, where a,b,c, and d are unknown positive integers. The reaction mush be balanced; that is, the number of atoms of each element must be the same before and after the reaction. For example, because the number of oxygen atoms must remain the same, 10a=8d. While there are many possible choices for a,b,c, and d that balance the reaction, it is customary to use the smallest possible integers. Balance this reaction. a= b= c= d=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


