Question: solve using unsteady state material balance equation for A Q2: In an enzyme-catalyzed reaction with stoichiometry A B. A is consumed at a rate given
solve using unsteady state material balance equation for A
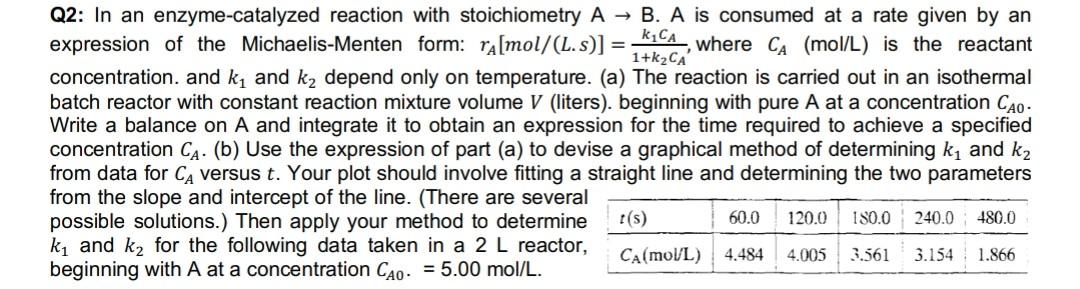
Q2: In an enzyme-catalyzed reaction with stoichiometry A B. A is consumed at a rate given by an expression of the Michaelis-Menten form: ra[mol/(L. s)] = k1CA, where CA (mol/L) is the reactant 1+k2CA concentration, and k1 and ky depend only on temperature. (a) The reaction is carried out in an isothermal batch reactor with constant reaction mixture volume V (liters). beginning with pure A at a concentration Cao- Write a balance on A and integrate it to obtain an expression for the time required to achieve a specified concentration CA. (b) Use the expression of part (a) to devise a graphical method of determining ky and kz from data for CA versus t. Your plot should involve fitting a straight line and determining the two parameters from the slope and intercept of the line. (There are several possible solutions.) Then apply your method to determine t(s) 60.0 120.0 180.0 240.0 ki and kz for the following data taken in a 2 L reactor, CA(mol/L) 4.484 4.005 3.561 3.154 1.866 beginning with A at a concentration CAO. = 5.00 mol/L. 480.0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


