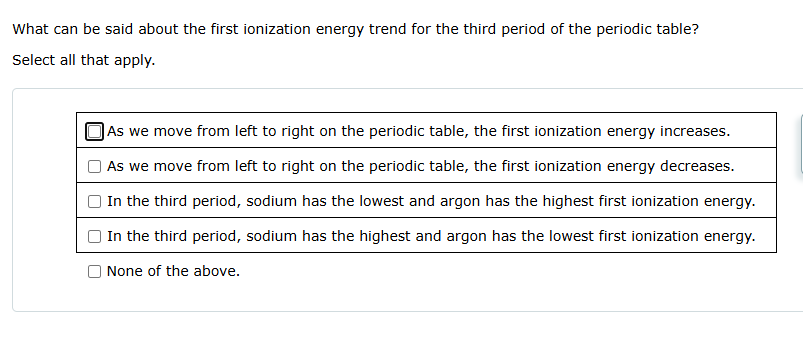
Question: SOLVE What can be said about the first ionization energy trend for the third period of the periodic table? Select all that apply. (As we
SOLVE
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


