Question: Some SO3 is placed in a sealed flask and heated to 1280K. When equilibrium is reached, the flask is found to contain SO3(1.21102M),SO2(2.96102M), and O2(3.56102M).
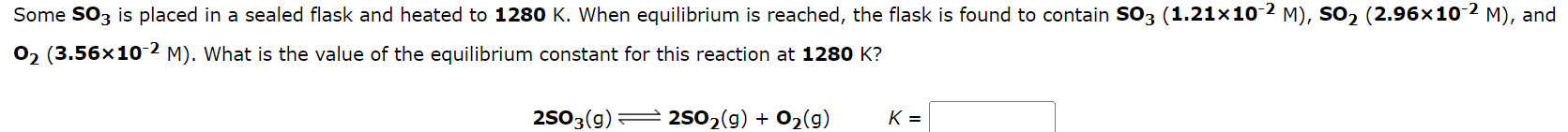
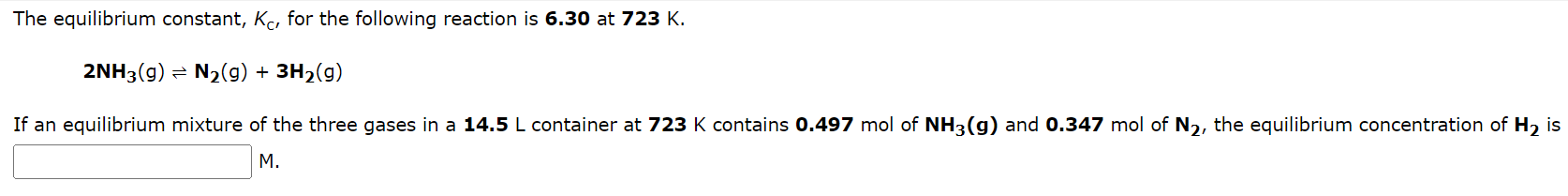
Some SO3 is placed in a sealed flask and heated to 1280K. When equilibrium is reached, the flask is found to contain SO3(1.21102M),SO2(2.96102M), and O2(3.56102M). What is the value of the equilibrium constant for this reaction at 1280K ? 2SO3(g)2SO2(g)+O2(g)K= The equilibrium constant, KC, for the following reaction is 6.30 at 723K. 2NH3(g)N2(g)+3H2(g) If an equilibrium mixture of the three gases in a 14.5L container at 723K contains 0.497mol of NH3(g) and 0.347mol of N2, the equilibrium concentration of H2 is M
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


