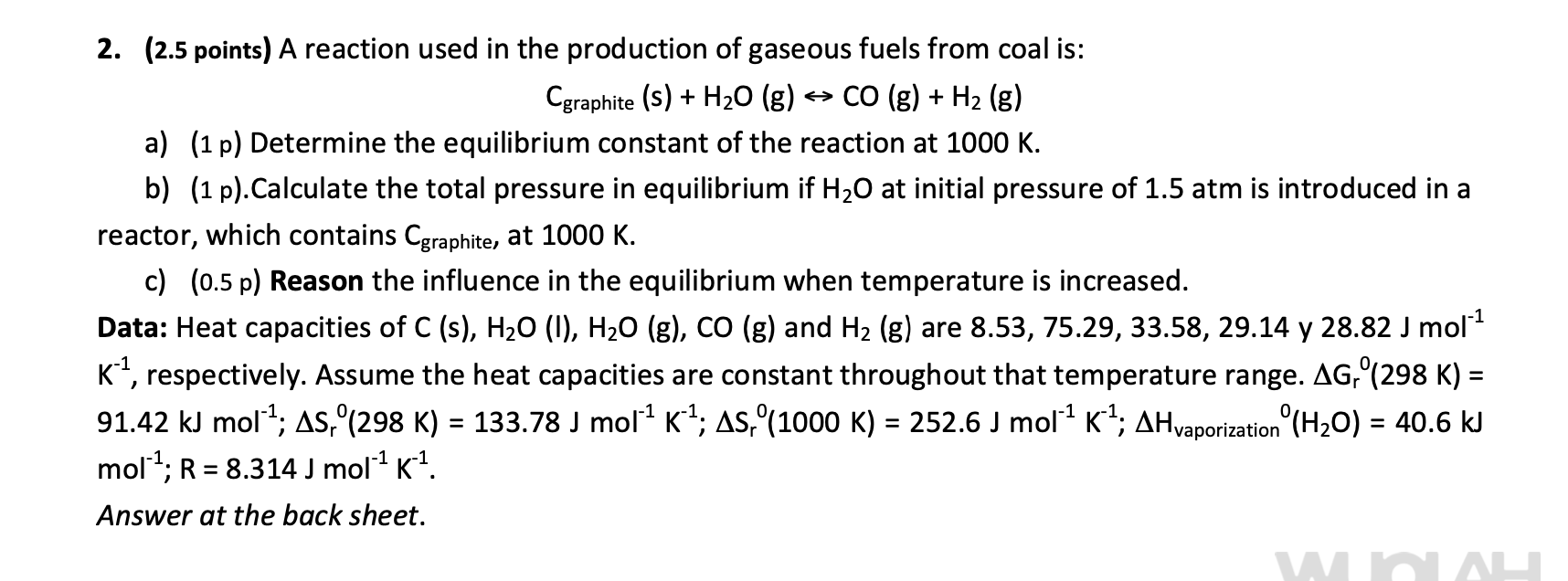
Question: Someone can help me to solve part b) ? 2. (2.5 points) A reaction used in the production of gaseous fuels from coal is: Cgraphite
 Someone can help me to solve part b) ?
Someone can help me to solve part b) ?
2. (2.5 points) A reaction used in the production of gaseous fuels from coal is: Cgraphite (s) + H20 (g) => CO (g) + H2 (g) a) (1 p) Determine the equilibrium constant of the reaction at 1000 K. b) (1 p).Calculate the total pressure in equilibrium if H20 at initial pressure of 1.5 atm is introduced in a reactor, which contains Cgraphite, at 1000 K. c) (0.5 p) Reason the influence in the equilibrium when temperature is increased. Data: Heat capacities of C (s), H20 (1), H20 (g), CO (g) and H2 (g) are 8.53, 75.29, 33.58, 29.14 y 28.82 J mol1 K ?, respectively. Assume the heat capacities are constant throughout that temperature range. AG,(298 K) = 91.42 kJ mol-4; 48,(298 K) = 133.78 J mor? K4; AS,(1000 K) = 252.6 J moll K4; AHvaporization (H20) = 40.6 kJ mol?; R = 8.314 J molK1. Answer at the back sheet. = 1 = = = , , , =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


