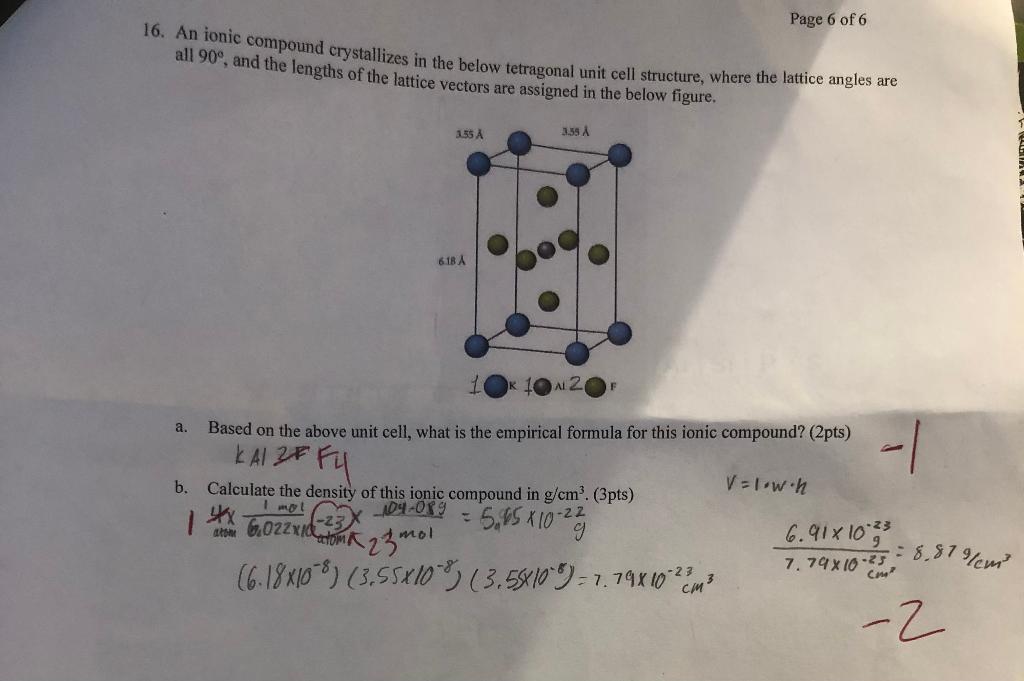
Question: someone help I cant figure out what I did wrong 16. An ionic compound crystallizes in the below tetragonal unit cell structure, where the lattice
 someone help I cant figure out what I did wrong
someone help I cant figure out what I did wrong
16. An ionic compound crystallizes in the below tetragonal unit cell structure, where the lattice angles are all 90, and the lengths of the lattice vectors are assigned in the below figure. a. Based on the above unit cell, what is the empirical formula for this ionic compound? (2pts) KAl ZFFU b. Calculate the density of this ionic compound in g/cm3. (3pts) V=1wh
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


