Question: Sometimes a species in a given oxidation state undergoes self-oxidation reduction reaction (disproportionation) in aqueous medium to give products of lower and higher oxidation
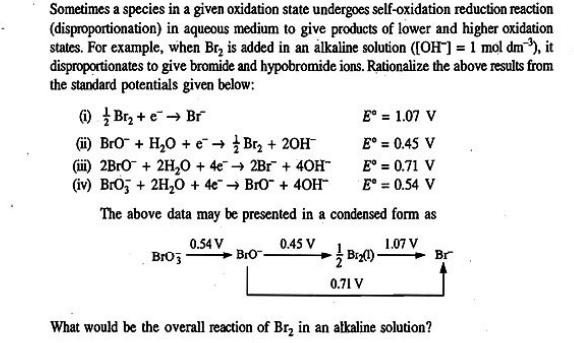
Sometimes a species in a given oxidation state undergoes self-oxidation reduction reaction (disproportionation) in aqueous medium to give products of lower and higher oxidation states. For example, when Br is added in an alkaline solution ([OH-] = 1 mol dm), it disproportionates to give bromide and hypobromide ions. Rationalize the above results from the standard potentials given below: (1) Br+e Br (ii) BrO + HO + (iii) 2BrO + 2HO + 4e (iv) BrO3 + 2HO + 4e Br + 20H 2Br + 40H BrO + 40H The above data may be presented in a condensed form as 0.54 V 1.07 V BrO3 Bro. 0.45 V E = 1.07 V E = 0.45 V E = 0.71 V E = 0.54 V + B(1) 0.71 V What would be the overall reaction of Br in an alkaline solution? Br
Step by Step Solution
3.42 Rating (155 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


