Question: soon as posable plese Identify the Uimiting reactant in the reaction of diphosphorus pentoxide and water to form H3PO4, if 24.5g of P2O5 and 12.9
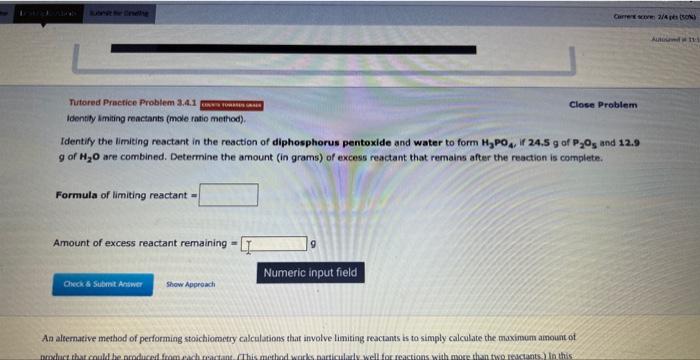
Identify the Uimiting reactant in the reaction of diphosphorus pentoxide and water to form H3PO4, if 24.5g of P2O5 and 12.9 g of H2O are combined. Determine the amount (in grams) of excess reactant that remains after the reaction is cornplete. Formula of limiting reactant = Amount of excess reactant remaining = Aa altemative method of performing stoichiometry calculations that involve limiting reactants ts to simply calculate the maximaum ainount of
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


