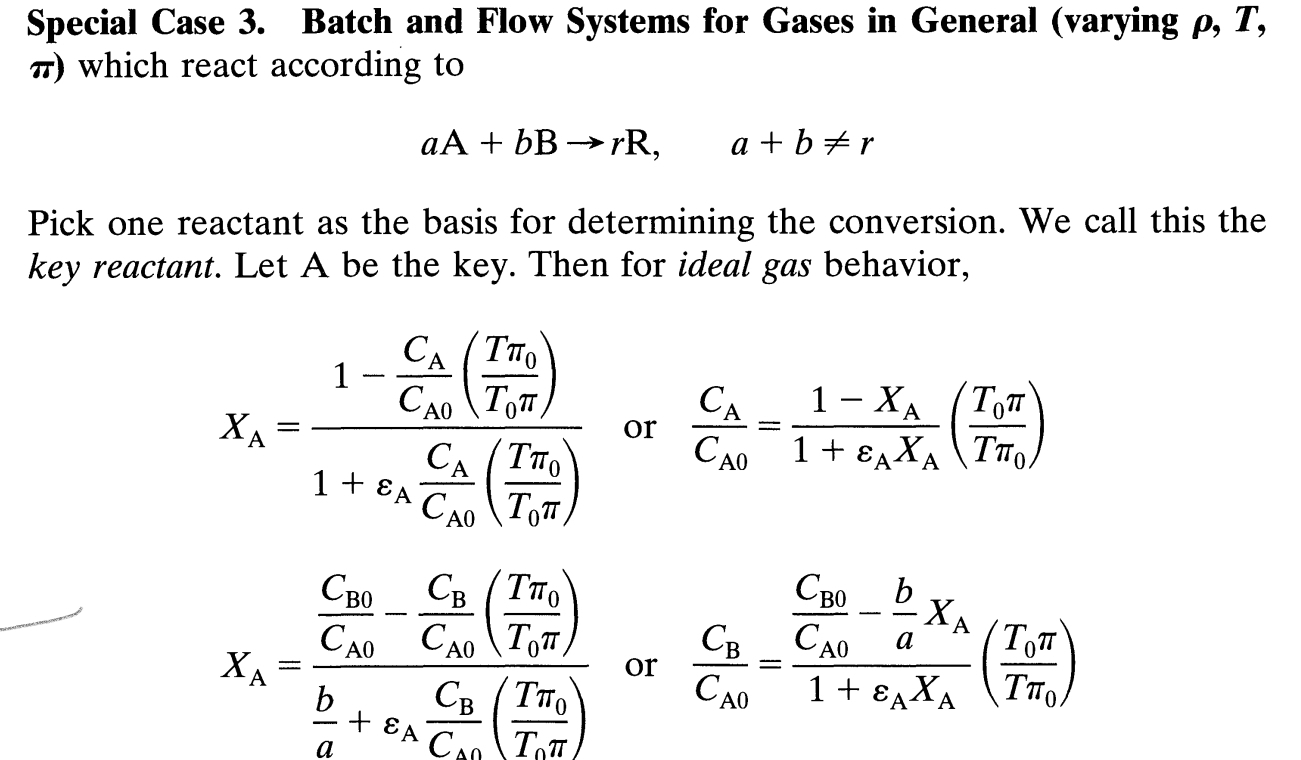
Question: Special Case 3 . Batch and Flow Systems for Gases in General ( varying , T , which react according to a A + b
Special Case Batch and Flow Systems for Gases in General varying
which react according to
Pick one reactant as the basis for determining the conversion. We call this the
key reactant. Let A be the key. Then for ideal gas behavior,
How can I dervice xa and CACA
is it came from ideal gas equation?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


