Question: specifically d and e Use the following data table to answer the following questions. a. The order of reaction with respect to concentration of A
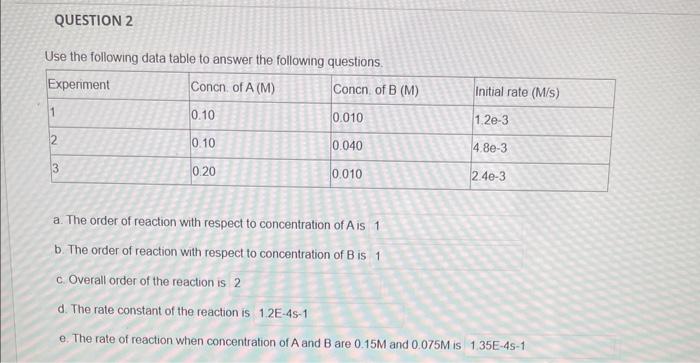
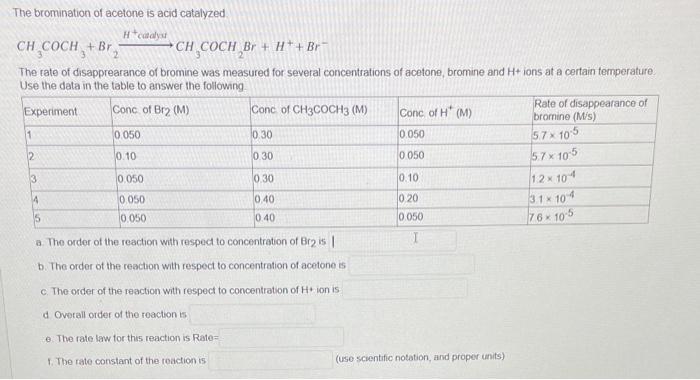
Use the following data table to answer the following questions. a. The order of reaction with respect to concentration of A is b. The order of reaction with respect to concentration of B is c. Overall order of the reaction is 2 d. The rate constant of the reaction is e. The rate of reaction when concentration of A and B are 0.15M and 0.075M is The bromination of acetone is acid catalyzed CH3COCH3+Br2H+cadyarCH3COCH2Br+H++Br The rate of disapprearance of bromine was measured for several concentrations of acetone, bromine and H+ ions at a certain temperature Use the data in the table to answer the following. a. The order of the reaction with respect to concentration of Bre is b. The order of the reaction with respect to concentration of acetone is c. The order of the reaction with respect to concentration of H+ ion is d Ovirall order of the reaction is e. The rate law tor this reaction is Rate= 1. The rate constant of the reaction is (use scientific notation, and proper units)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


