Question: Specify the hybridization (sp, sp^2 ,sp^4 ,sp^2d, sp^3d, spd^2 etc.) of the circled atoms in the structures below (for your convenience, lone pairs are already

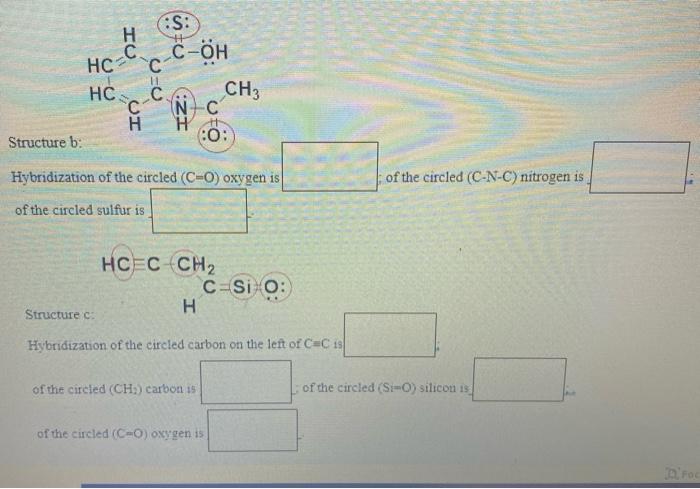
7. Specify the hybridization (sp. sp. sp. sp?d, spd, spd etc.) of the circled atoms in the structures below (for your convenience, lone pairs are already shown): (0.75 pts, 0.25 pt each) CH3 HNO CH2 CH H3C CH Structure a: Hybridization of the circled carbon is of the circled nitrogen is :S: C-OH H C HC HC 11 . UI CH3 INC H 10: Structure b: Hybridization of the circled (C=C) oxygen is of the circled (C-N-C) nitrogen is of the circled sulfur is HCEC CH2 C-Si O: H Structure c: Hybridization of the circled carbon on the left of C C 18 of the circled (CH) carbon 15 of the circled (Si-O) silicon 15 of the circled (CO) oxygen is Foc
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


