Question: SPP3701 Minor Test 01 2022 QUESTION 1 In a batch still distillation column, a mixture of 2 000 mol mol% CH44 000 mol% of C2H4,
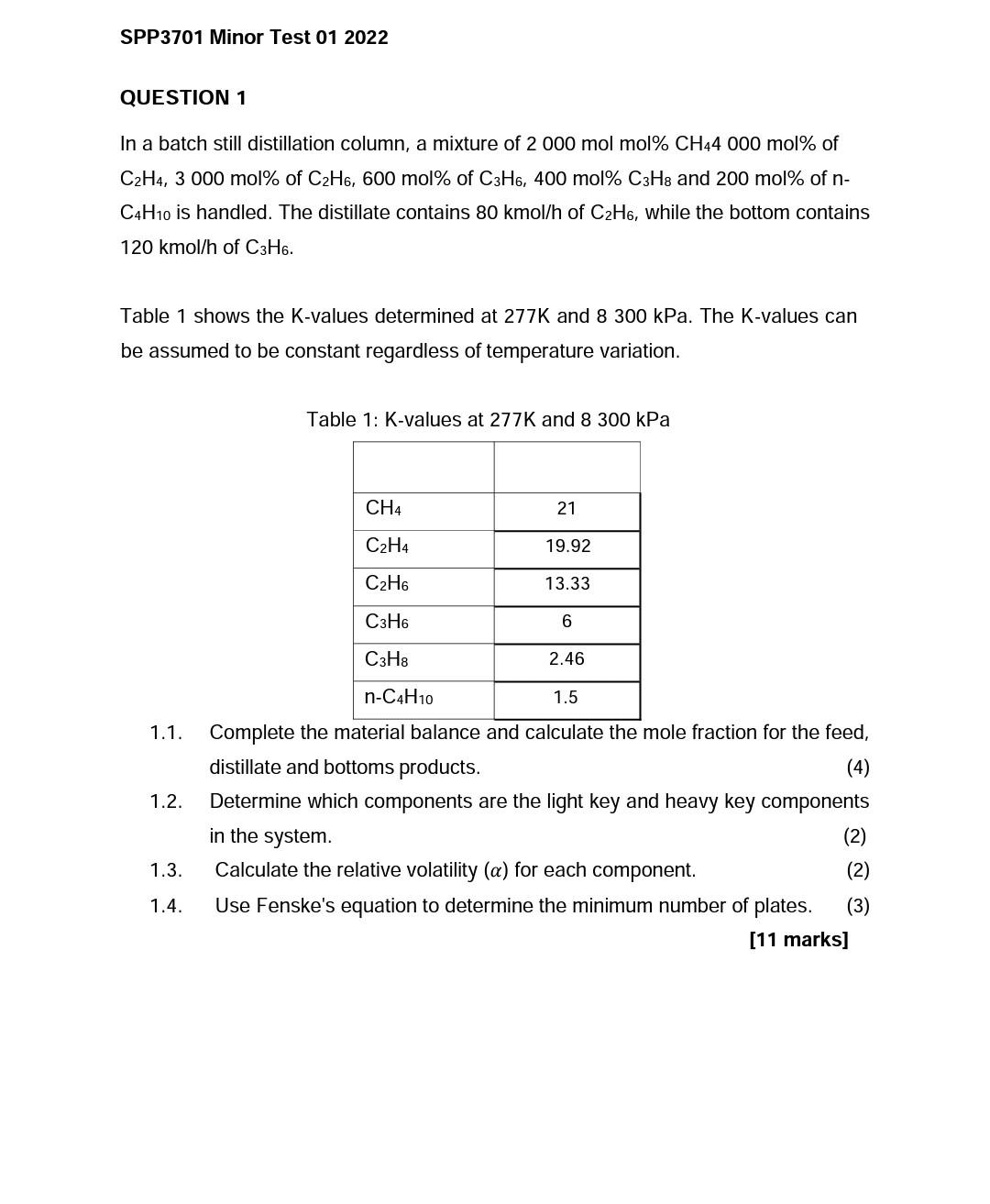
SPP3701 Minor Test 01 2022 QUESTION 1 In a batch still distillation column, a mixture of 2 000 mol mol% CH44 000 mol% of C2H4, 3 000 mol% of C2H6, 600 mol% of C3H6, 400 mol% C3H8 and 200 mol% of n- C4H10 is handled. The distillate contains 80 kmol/h of C2H6, while the bottom contains 120 kmol/h of C3H6. Table 1 shows the K-values determined at 277K and 8 300 kPa. The K-values can be assumed to be constant regardless of temperature variation. Table 1: K-values at 277K and 8 300 kPa CH4 21 C2H4 19.92 C2H6 13.33 C3H6 6 C3H8 2.46 n-C4H10 1.5 1.1. 1.2. Complete the material balance and calculate the mole fraction for the feed, distillate and bottoms products. (4) Determine which components are the light key and heavy key components in the system. (2) Calculate the relative volatility (a) for each component. (2) Use Fenske's equation to determine the minimum number of plates. (3) [11 marks] 1.3. 1.4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


