Question: SrTiO3 The structure factor represents the scattering of x-rays from the periodic arrangement of atoms within a particular crystal structure. Using the atomic positions for
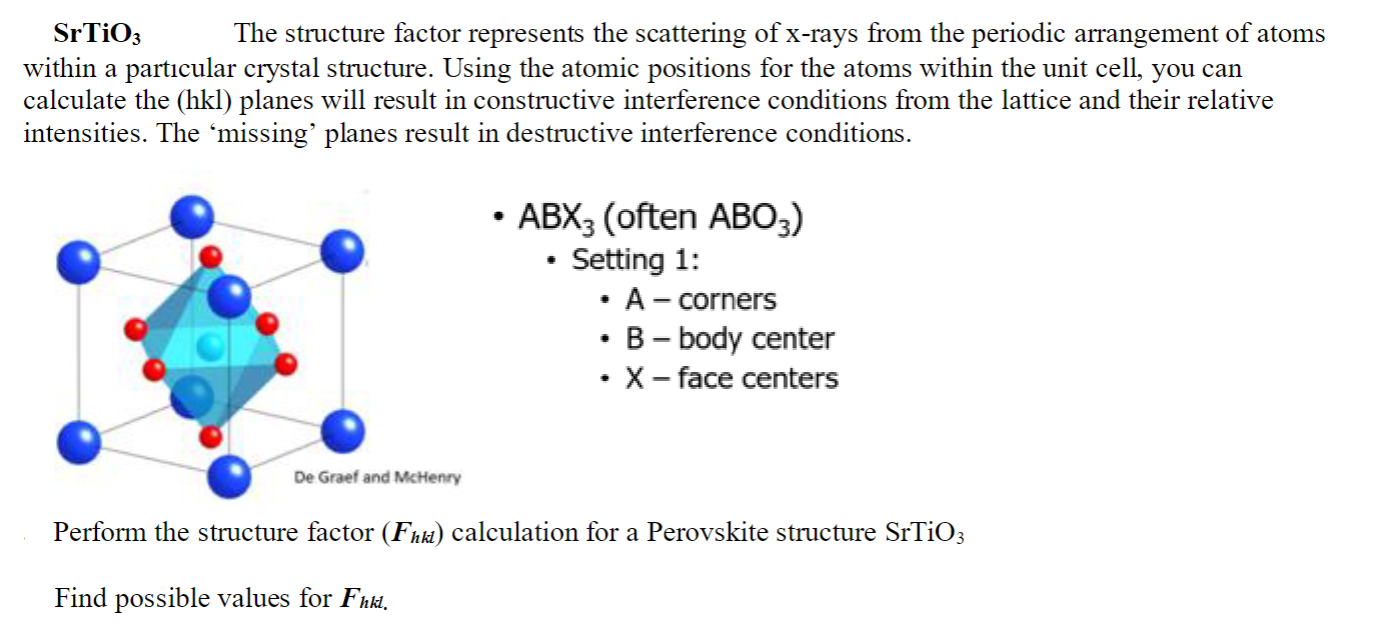
SrTiO3 The structure factor represents the scattering of x-rays from the periodic arrangement of atoms within a particular crystal structure. Using the atomic positions for the atoms within the unit cell, you can calculate the (hkl) planes will result in constructive interference conditions from the lattice and their relative intensities. The 'missing' planes result in destructive interference conditions. - ABX3 (often ABO3 ) - Setting 1: - A - corners - B - body center - X - face centers Perform the structure factor (Fhkl) calculation for a Perovskite structure SrTiO3 Find possible values for Fhkt
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


