Question: standard conditions Consider this reaction: Express your answer using three signific I2(g)+Cl2(g)2ICl(g)K=6.91103at460K Calculate PG for the reaction at 460K under each of the following conditions:
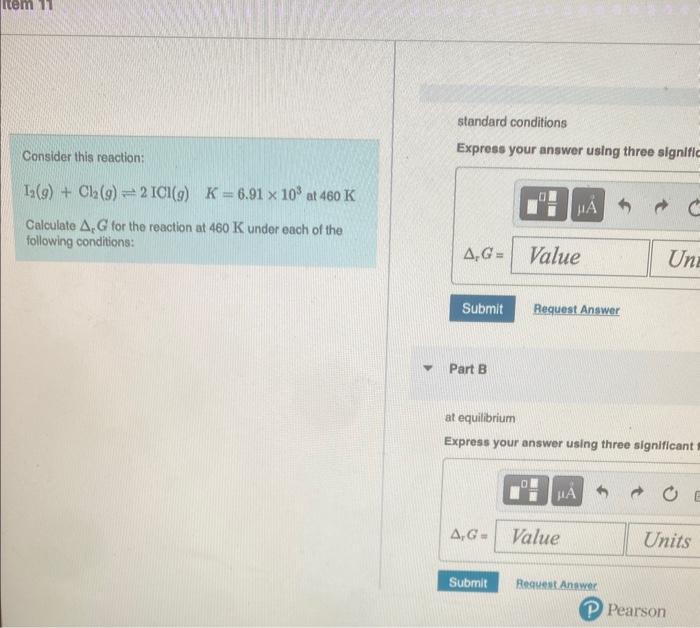
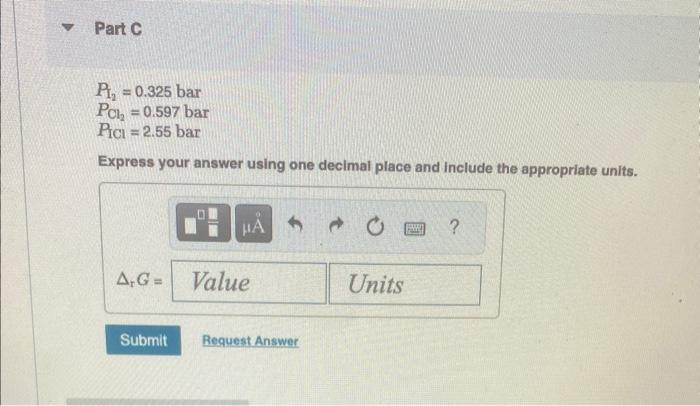
standard conditions Consider this reaction: Express your answer using three signific I2(g)+Cl2(g)2ICl(g)K=6.91103at460K Calculate PG for the reaction at 460K under each of the following conditions: Part B at equilibrium Express your answer using three significant P12=0.325barPCl2=0.597barPICl=2.55bar Express your answer using one decimal place and include the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


