Question: Statement Liquid octane ( C 8 H 1 8 ) at 2 5 C , 1 atm and a mass flow rate of 0 .
Statement
Liquid octane at atm and a mass flow rate of enters an internal
combustion engine operating at steady state. The fuel burns with air entering the engine in
a separate stream at atm. Combustion products exit at atm with a dry
molar analysis of and The engine develops
power at the rate of kW
Determine
a the balanced reaction equation.
b the rate of heat transfer from the engine, in kW
c an exergetic efficiency for the engine.
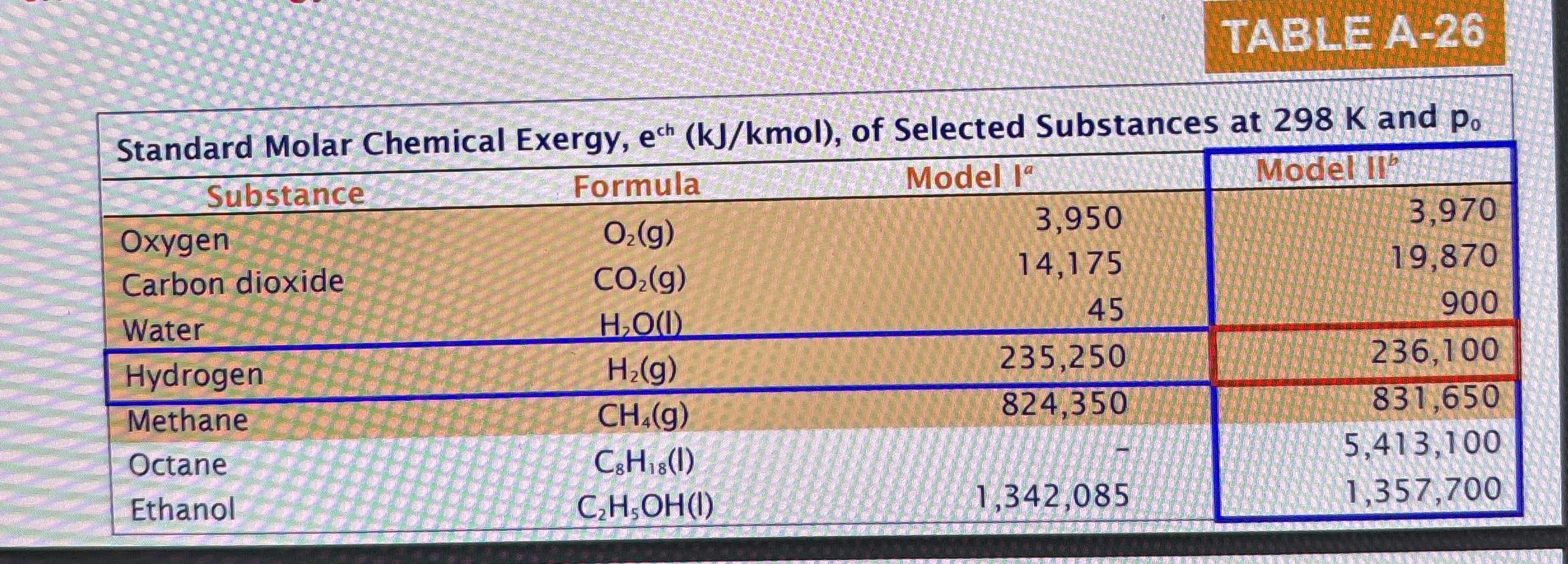
Use the environment of Table AModel II
tableStandard Molar Chemical Exergy, mol of Selected Substances at K and,Model I Model l and neglect the effects of motion and gravity.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


