Question: STATEMENT OF THE PROBLEM Question No. 7 Consider a concentrated hydrochloric acid with the following specifications: 37.0%(w/w)HCl density of the reagent is 1.19g/mL begin{tabular}{|l|ll|} hline
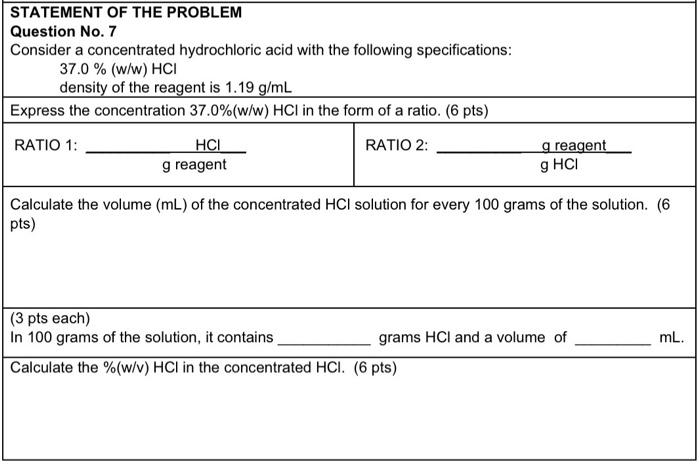
STATEMENT OF THE PROBLEM Question No. 7 Consider a concentrated hydrochloric acid with the following specifications: 37.0%(w/w)HCl density of the reagent is 1.19g/mL \begin{tabular}{|l|ll|} \hline Express the concentration 37.0%(w/w)HCl in the form of a ratio. (6 pts) \\ \hline RATIO 1: greagentHCl & RATIO 2: & g reagent \\ \cline { 4 - 4 } & & gHCl \\ \hline \end{tabular} Calculate the volume (mL ) of the concentrated HCl solution for every 100 grams of the solution. (6 pts) (3 pts each) In 100 grams of the solution, it contains grams HCl and a volume of mL. Calculate the %(w/v)HCl in the concentrated HCl. (6 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


