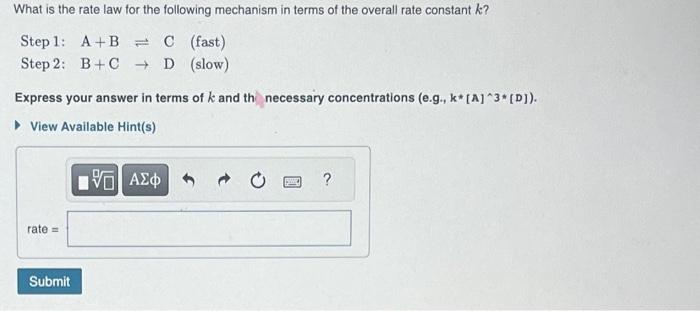
Question: Step 1: A+BC (fast) Step 2: B +CD (slow) Express your answer in terms of k and th necessary concentrations (e.g., k[A]3[D]). View Available Hint(s)
![answer in terms of k and th necessary concentrations (e.g., k[A]3[D]). View](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f9046de4fca_62966f9046d86046.jpg)
Step 1: A+BC (fast) Step 2: B +CD (slow) Express your answer in terms of k and th necessary concentrations (e.g., k[A]3[D]). View Available Hint(s) Consider the reaction 2XY2+Z22XY2Z which has a rate law of rate=k[XY2][Z2] Select a possible mechanism for the reaction. View Available Hint(s) Step 1: Z2Z+Z (slow) A Step 2: XY2+ZXY2Z (fast) Step 3: XY2+ZXY2Z (fast) Step 1: XY2+Z2XY2Z+Z (slow) Step 2: XY2+ZXY2Z (fast) Step 1: XY2+Z2XY2Z2 (slow) Step 2: XY2Z2XY2Z+Z (fast) Step 1: 2XY2X2Y4 (fast) Step 2: X2Y4+Z22XY2Z (slow) EStep1:2XY2+Z22XY2Z(slow)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


