Question: Strontium difluoride (SrF2) has the cubic fluorite structure, shown below, with a lattice parameter of a=0.578 nm. Aluminum (Al) is a face-centered cubic (also shown
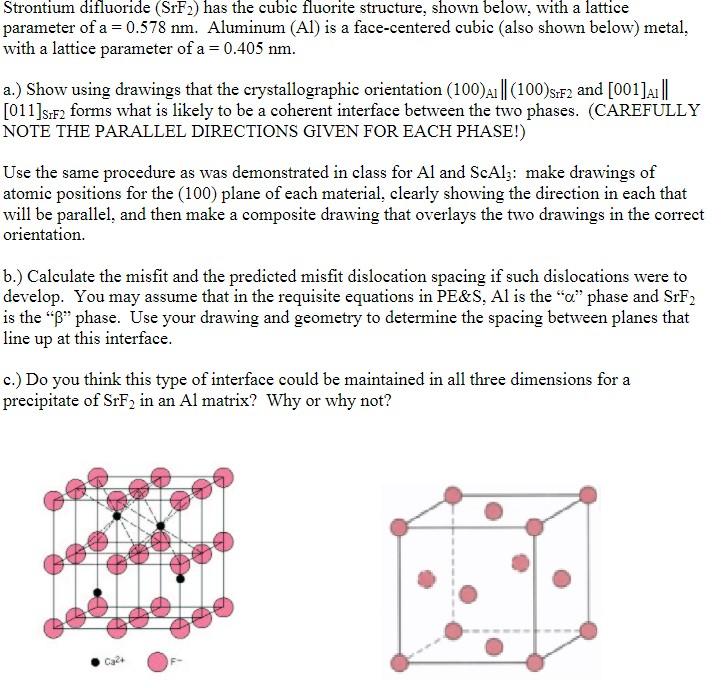
Strontium difluoride (SrF2) has the cubic fluorite structure, shown below, with a lattice parameter of a=0.578 nm. Aluminum (Al) is a face-centered cubic (also shown below) metal, with a lattice parameter of a = 0.405 nm. a.) Show using drawings that the crystallographic orientation (100) A1 ||(100)s:F2 and [001]A1 ||| [011]s:F2 forms what is likely to be a coherent interface between the two phases. (CAREFULLY NOTE THE PARALLEL DIRECTIONS GIVEN FOR EACH PHASE!) Use the same procedure as was demonstrated in class for Al and ScAlz: make drawings of atomic positions for the (100) plane of each material, clearly showing the direction in each that will be parallel, and then make a composite drawing that overlays the two drawings in the correct orientation. b.) Calculate the misfit and the predicted misfit dislocation spacing if such dislocations were to develop. You may assume that in the requisite equations in PE&S, Al is the "c" phase and SrF2 is the "B" phase. Use your drawing and geometry to determine the spacing between planes that line up at this interface. c.) Do you think this type of interface could be maintained in all three dimensions for a precipitate of SrF2 in an Al matrix? Why or why not? Ca24 Strontium difluoride (SrF2) has the cubic fluorite structure, shown below, with a lattice parameter of a=0.578 nm. Aluminum (Al) is a face-centered cubic (also shown below) metal, with a lattice parameter of a = 0.405 nm. a.) Show using drawings that the crystallographic orientation (100) A1 ||(100)s:F2 and [001]A1 ||| [011]s:F2 forms what is likely to be a coherent interface between the two phases. (CAREFULLY NOTE THE PARALLEL DIRECTIONS GIVEN FOR EACH PHASE!) Use the same procedure as was demonstrated in class for Al and ScAlz: make drawings of atomic positions for the (100) plane of each material, clearly showing the direction in each that will be parallel, and then make a composite drawing that overlays the two drawings in the correct orientation. b.) Calculate the misfit and the predicted misfit dislocation spacing if such dislocations were to develop. You may assume that in the requisite equations in PE&S, Al is the "c" phase and SrF2 is the "B" phase. Use your drawing and geometry to determine the spacing between planes that line up at this interface. c.) Do you think this type of interface could be maintained in all three dimensions for a precipitate of SrF2 in an Al matrix? Why or why not? Ca24
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


