Question: struggling with these two! At a particular temperature, the decomposition of dinitrogen tetraoxide into nitrogen dioxide has an equilibrium constant of 17. N2O4(g)2NO2(g) If 2.2mol
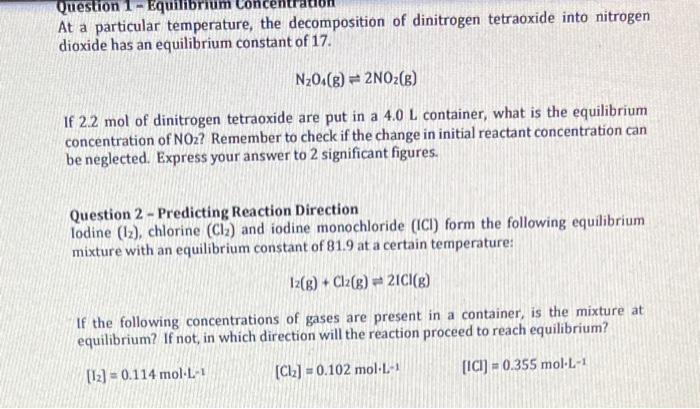
At a particular temperature, the decomposition of dinitrogen tetraoxide into nitrogen dioxide has an equilibrium constant of 17. N2O4(g)2NO2(g) If 2.2mol of dinitrogen tetraoxide are put in a 4.0L container, what is the equilibrium concentration of NO2 ? Remember to check if the change in initial reactant concentration can be neglected. Express your answer to 2 significant figures. Question 2 - Predicting Reaction Direction lodine (I2), chlorine (Cl2) and iodine monochloride (ICI) form the following equilibrium mixture with an equilibrium constant of 81.9 at a certain temperature: I2(g)+Cl2(g)2ICl(g) If the following concentrations of gases are present in a container, is the mixture at equilibrium? If not, in which direction will the reaction proceed to reach equilibrium? [I2]=0.114molL1 [Cl2]=0.102molL1 [ICl]=0.355molL1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


