Question: Students should use the information found in Experiment 23 (pgs 25-36) of their CH132L Laboratory Manual to complete Table 1. Each sample was made with
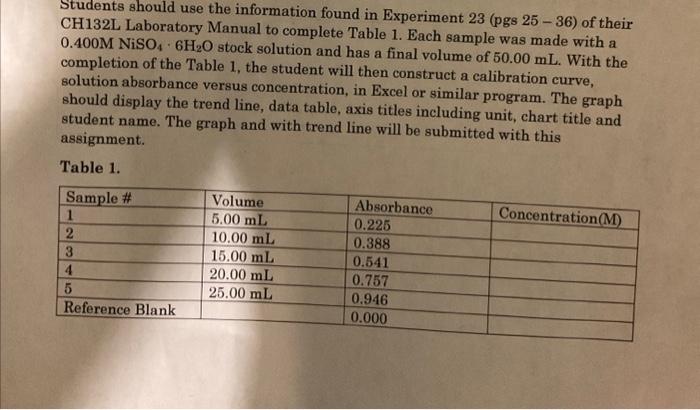


Students should use the information found in Experiment 23 (pgs 25-36) of their CH132L Laboratory Manual to complete Table 1. Each sample was made with a 0.400MNiSO46H2O stock solution and has a final volume of 50.00mL. With the completion of the Table 1 , the student will then construct a calibration curve, solution absorbance versus concentration, in Excel or similar program. The graph should display the trend line, data table, axis titles including unit, chart title and student name. The graph and with trend line will be submitted with this assignment. Table 1. Using the calibration curve constructed from the data in Table 1. The student will solve the following problems. All work and calculation must be shown in order to receive credit for the work. (1) A student determines that an unknown NiSO46H2O solution has an absorbance of 0.458. What is the concentration of the unknown NiSO4. 6H2O? ? Show All Calculations: (2) A student is give a solution of 0.147MNiSO46H2O. Determine the absorbance of the solution. Show All Calculations: (1) Why are using the correct significant figures important to this exercise? (2) Discuss an example of why the determination of the absorbance of a solution is important. Think real world. (3) Discuss the accuracy of using the trendline to calculate the concentration of the nickel solution versus simply "eye balling" the graphical data. (4) What are the most important parameters to pay attention to in this exercise? (5) How would inaccurate data collection affect the outcome of the experiment
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


