Question: Study This Chemical Reaction: Mg + Cl2 + MgCl2 Then, Write Balanced Half-Reactions Describing The Oxidation And Reduction That Happen In This Reaction. Oxidation: Reduction:
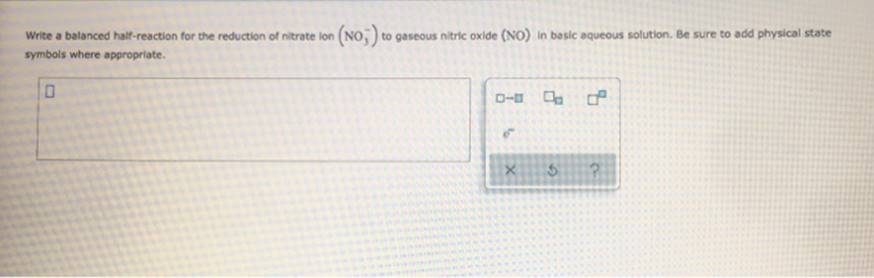
Study This Chemical Reaction: Mg + Cl2 + MgCl2 Then, Write Balanced Half-Reactions Describing The Oxidation And Reduction That Happen In This Reaction. Oxidation: Reduction: To ? 5 ? Write A Balanced Half-Reaction For The Reduction Of Nitrate Ion (No;) To Gaseous Nitric Oxide (NO) In Basic Aqueous Solution. Be Sure To Add Physical State Symbols Where
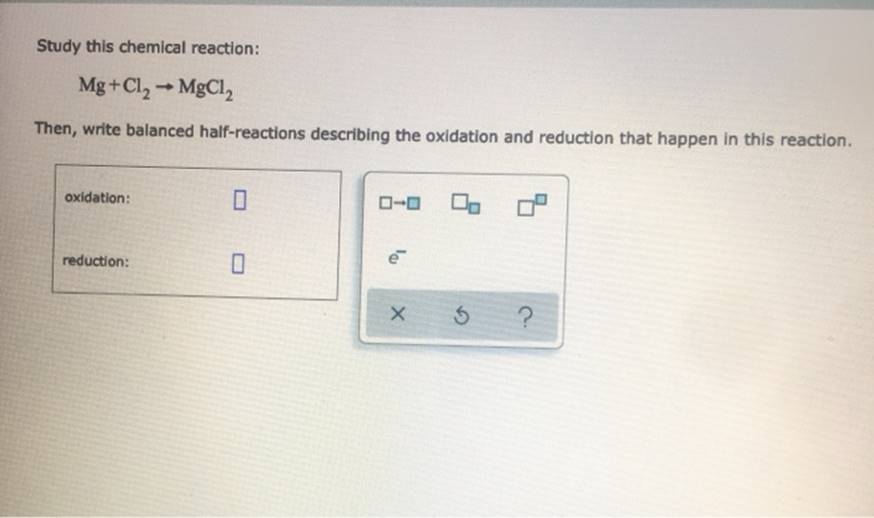
oxidation: reduction: To ? 5 ? Write a balanced half-reaction for the reduction of nitrate ion (no;) to gaseous nitric oxide (NO) In basic aqueous solution. Be sure to add physical state symbols where appropriate. ??? X 5
Study this chemical reaction: Mg+Cl MgCl2 Then, write balanced half-reactions describing the oxidation and reduction that happen in this reaction. oxidation: 00 reduction: X 5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


