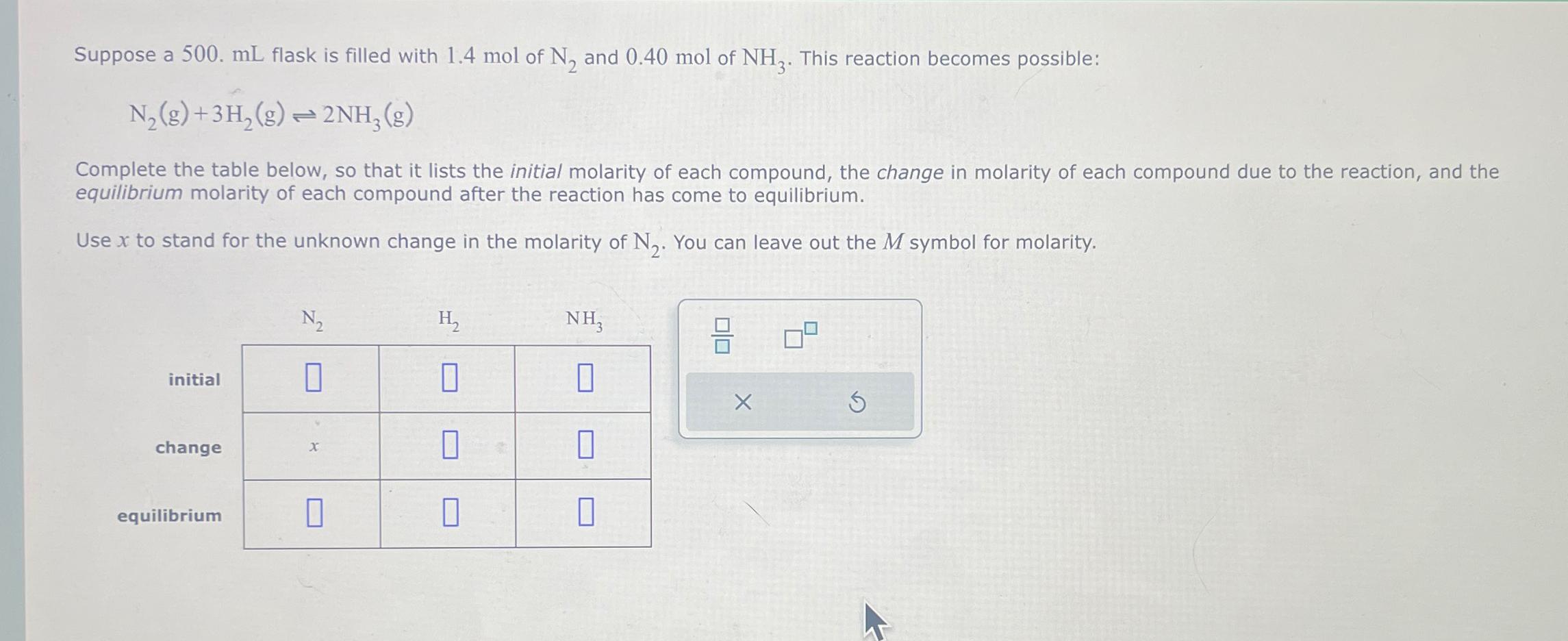
Question: Suppose a 5 0 0 . m L flask is filled with 1 . 4 mol of N 2 and 0 . 4 0 mol
Suppose a flask is filled with mol of and mol of This reaction becomes possible:
Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium.
Use to stand for the unknown change in the molarity of You can leave out the symbol for molarity.
table
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


