Question: Suppose a 500. ml flask is filled with 1.5 mol of SO, and 0.40 mol of SO3. This reaction becomes possible: 2502(g) + O2(g) =
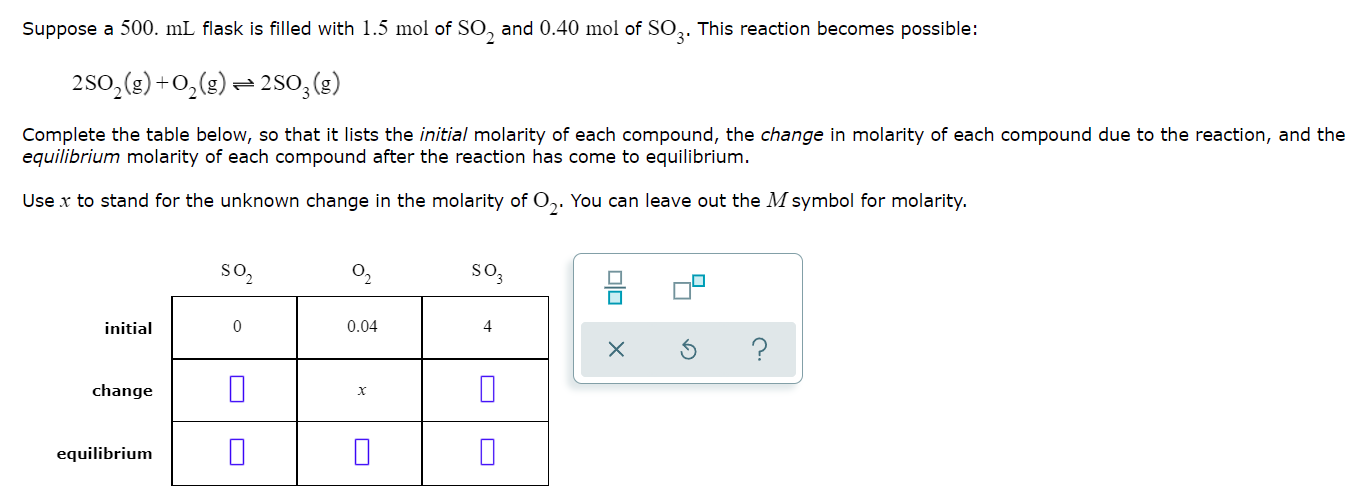

Suppose a 500. ml flask is filled with 1.5 mol of SO, and 0.40 mol of SO3. This reaction becomes possible: 2502(g) + O2(g) = 2503() Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of O2. You can leave out the M symbol for molarity. so2 02 soz : initial 0 0.04 4 ? change x . equilibrium a Suppose a 500. mL flask is filled with 0.40 mol of N2 and 1.8 mol of NO. The following reaction becomes possible: N2(g) + O2(g) = 2NO(g) The equilibrium constant K for this reaction is 7.65 at the temperature of the flask. Calculate the equilibrium molarity of N2. Round your answer to two decimal places
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


