Question: Suppose a battery was made using calcium (Ca) and bismuth (Bi) as the electrodes and had an operation temperature of 600 oC. Use the Ca-Bi
Suppose a battery was made using calcium (Ca) and bismuth (Bi) as the electrodes and had an operation temperature of 600 oC. Use the Ca-Bi phase diagram and standard reduction potentials for Bi3+/Bi and Ca2+/Ca to answer the following questions. Bi3+ + 3e- Bi; V0 = 0.308 V vs. NHE Ca2+ + 2e- Ca; V0 = -2.87 V vs. NHE
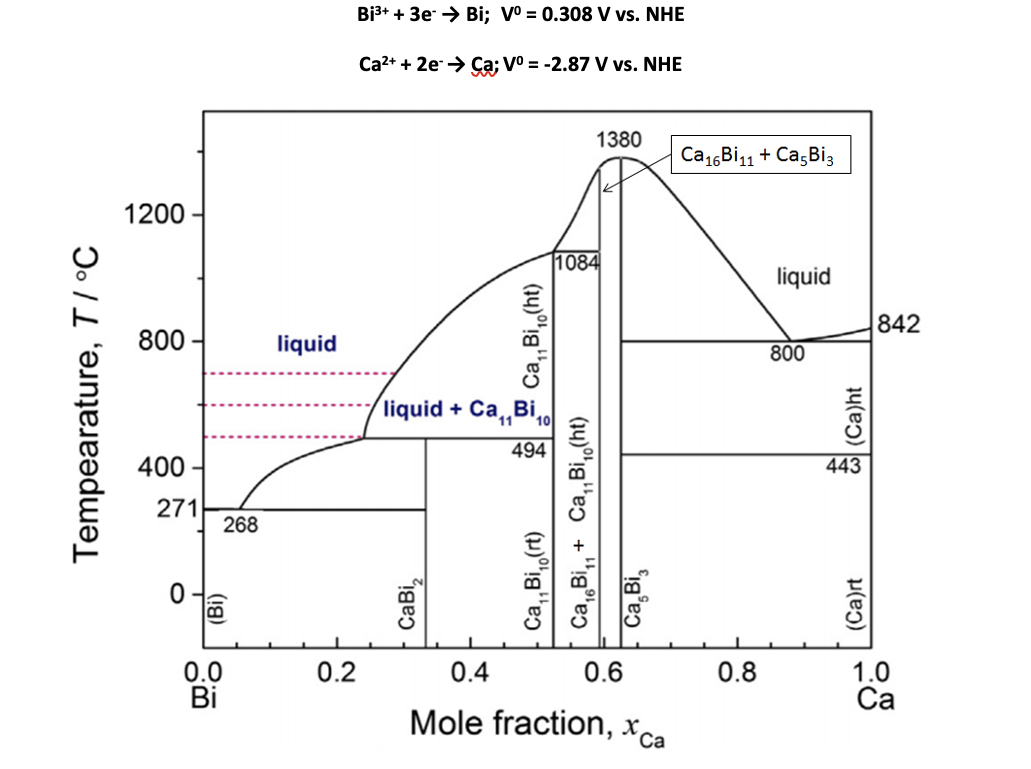
(a) Calculate the theoretical open circuit voltage for this battery at standard conditions. Then, draw the voltage profile for the discharge until the composition of the cathode reaches a mole fraction of xCa = 0.625.
(b) Suppose you took a snapshot of the battery after it has discharged until the composition of the cathode is xCa = 0.55. Label the different components of the battery at this point in the schematic in the electrochemical cell.
Bi3+ + 3e5 Bi; v = 0.308 V vs. NHE Ca2+ + 2e Ca; V = -2.87 V vs. NHE 1380 Ca 16Bi11 + Ca.Biz 1200 11084 liquid 800 842 liquid CaBi.Cht) 800 Tempearature, TIC liquid + Ca,, BIO 494 (ca)ht 400- 443 271 268 Ca, Big,+ Ca, Bio(ht) , Ca ,Biort) (BI) Ca.Biz (Ca)rt 0.2 0.4 0.0 0.6 0.8 1.0 Ca Mole fraction, x X ca
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


