Question: Suppose that you started with a liquid mixture that was 50mol% cyclopentane and 50 mol % 1,2-dimethoxyethane (glyme), whose approximate phase diagram is shown below
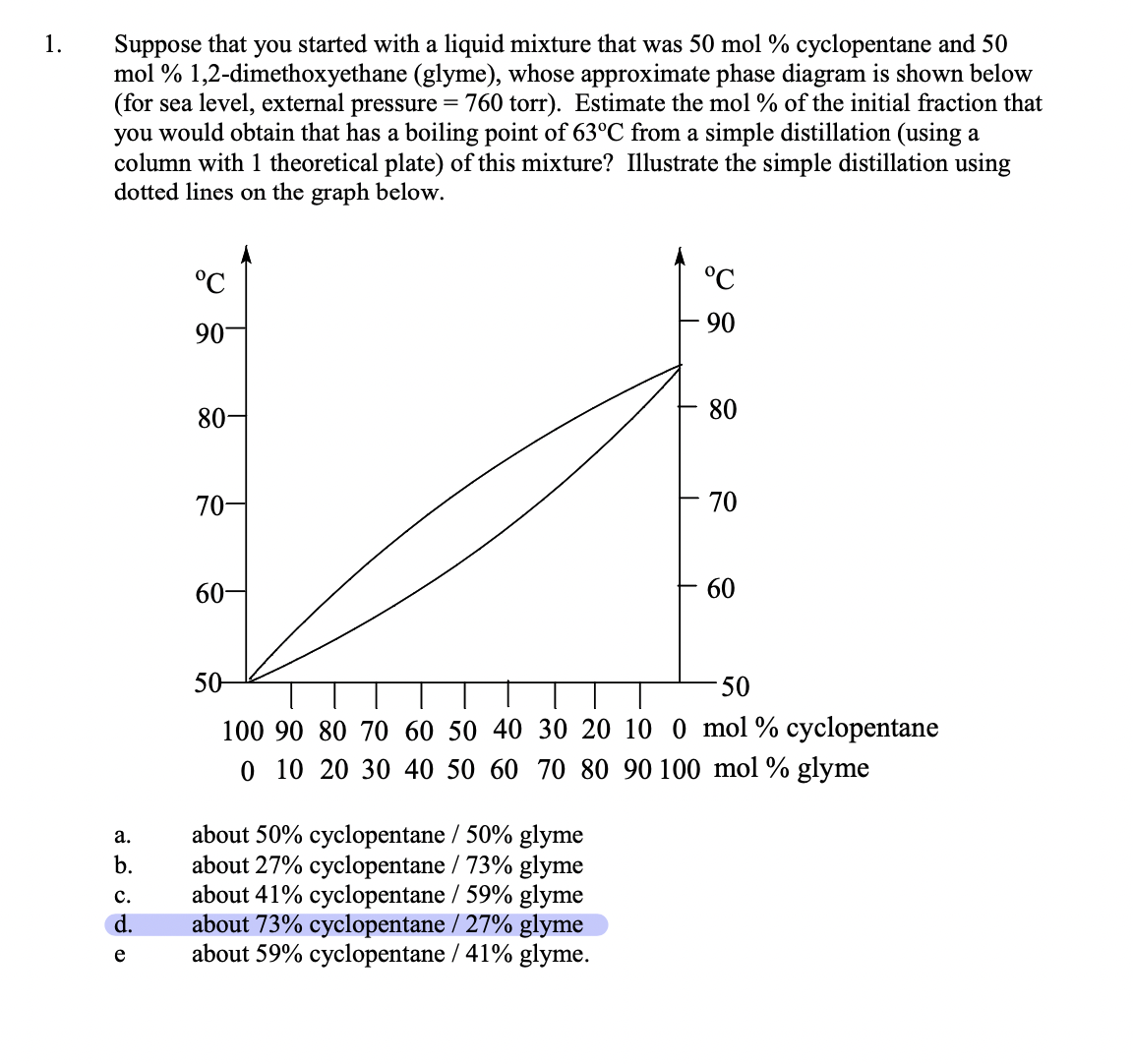
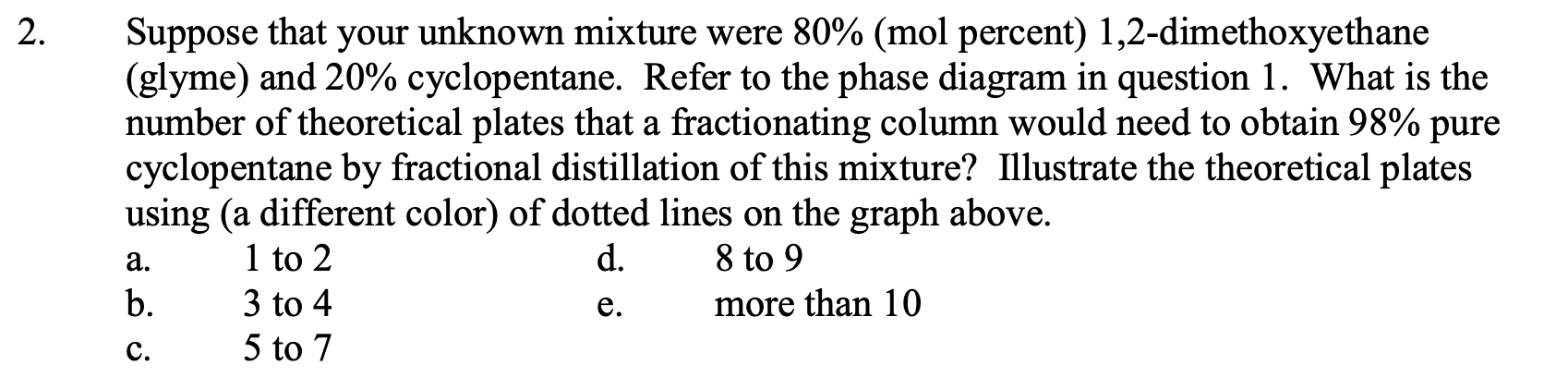
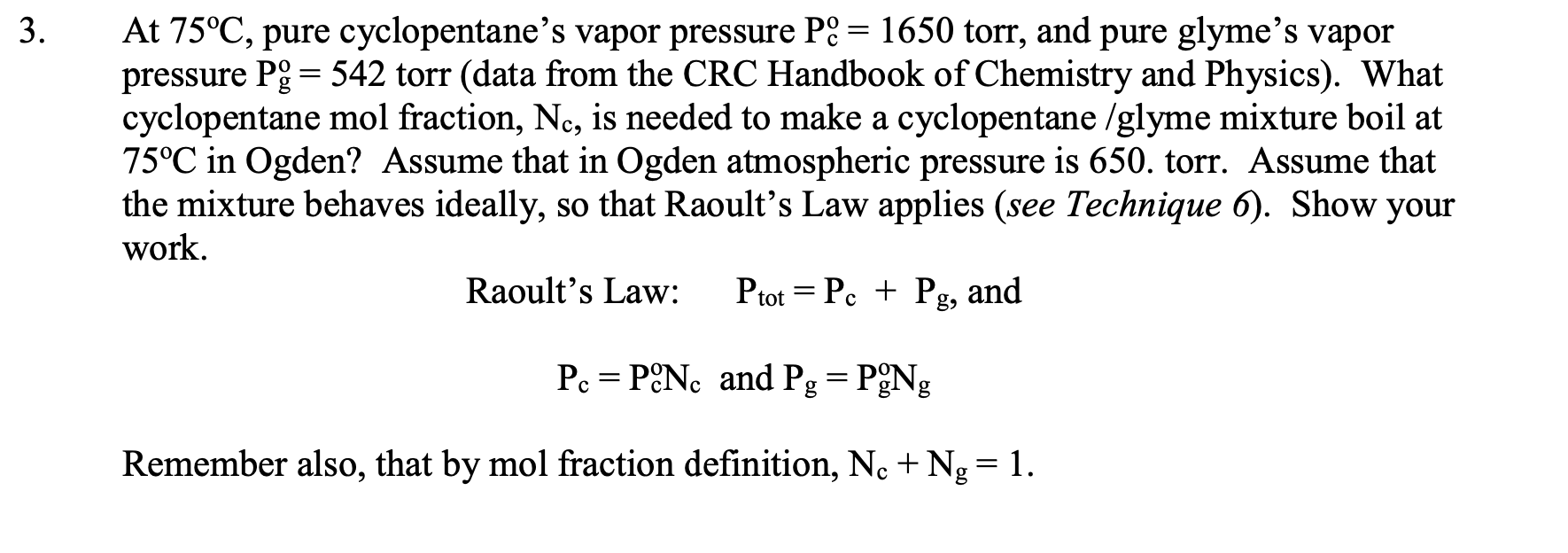
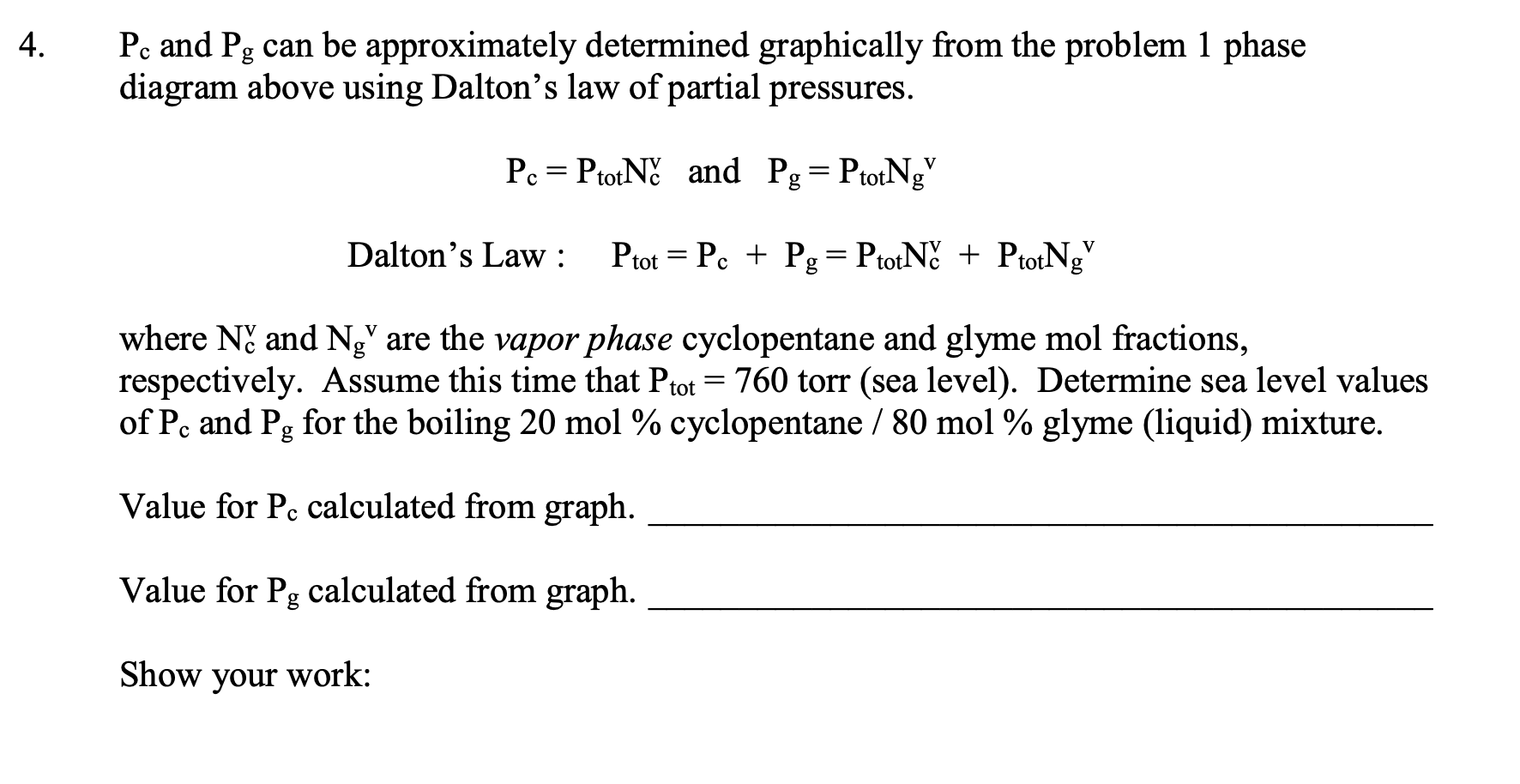
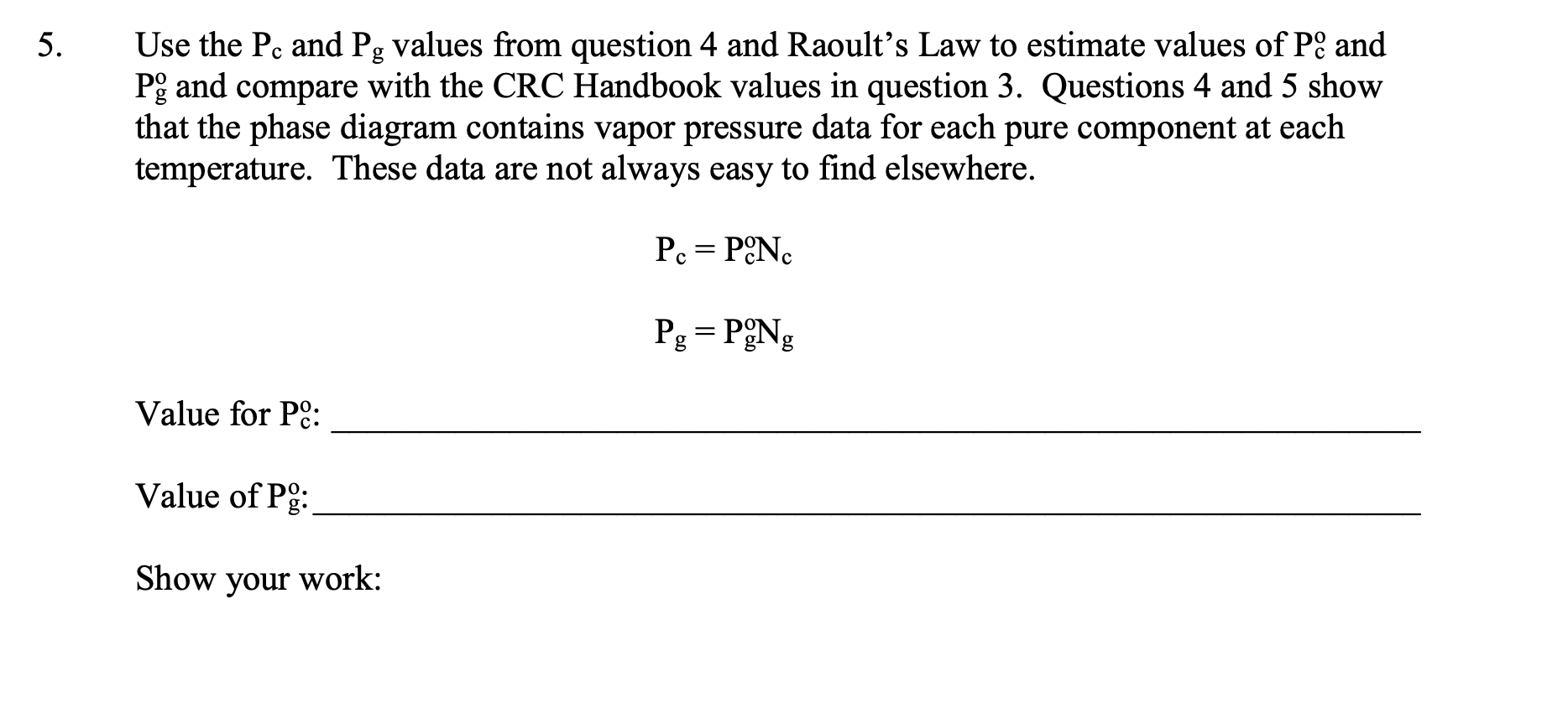
Suppose that you started with a liquid mixture that was 50mol% cyclopentane and 50 mol \% 1,2-dimethoxyethane (glyme), whose approximate phase diagram is shown below (for sea level, external pressure =760 torr). Estimate the mol \% of the initial fraction that you would obtain that has a boiling point of 63C from a simple distillation (using a column with 1 theoretical plate) of this mixture? Illustrate the simple distillation using dotted lines on the graph below. :yclopentane glyme a. about 50% cyclopentane / 50% glyme b. about 27% cyclopentane / 73% glyme c. about 41% cyclopentane / 59\% glyme d. about 73% cyclopentane / 27% glyme e about 59% cyclopentane / 41% glyme. Suppose that your unknown mixture were 80% (mol percent) 1,2-dimethoxyethane (glyme) and 20\% cyclopentane. Refer to the phase diagram in question 1. What is the number of theoretical plates that a fractionating column would need to obtain 98% pure cyclopentane by fractional distillation of this mixture? Illustrate the theoretical plates using (a different color) of dotted lines on the graph above. a. 1 to 2 d. 8 to 9 b. 3 to 4 e. more than 10 c. 5 to 7 At 75C, pure cyclopentane's vapor pressure Pco=1650 torr, and pure glyme's vapor pressure Pgo=542 torr (data from the CRC Handbook of Chemistry and Physics). What cyclopentane mol fraction, Nc, is needed to make a cyclopentane/glyme mixture boil at 75C in Ogden? Assume that in Ogden atmospheric pressure is 650. torr. Assume that the mixture behaves ideally, so that Raoult's Law applies (see Technique 6). Show your work. Raoult's Law: Ptot=Pc+Pg, and Pc=Pc0NcandPgg=Po0Ng Remember also, that by mol fraction definition, Nc+Ng=1. Pc and Pg can be approximately determined graphically from the problem 1 phase diagram above using Dalton's law of partial pressures. Pc=PtotNcvandPg=PtotNgv Dalton's Law : Ptot=Pc+Pg=PtotNcv+PtotNgv where Ncv and Ngv are the vapor phase cyclopentane and glyme mol fractions, respectively. Assume this time that Ptot=760 torr (sea level). Determine sea level values of Pc and Pg for the boiling 20mol% cyclopentane / 80mol% glyme (liquid) mixture. Value for Pc calculated from graph. Value for Pg calculated from graph. Show your work: Use the Pc and Pg values from question 4 and Raoult's Law to estimate values of Pco and Pggo and compare with the CRC Handbook values in question 3. Questions 4 and 5 show that the phase diagram contains vapor pressure data for each pure component at each temperature. These data are not always easy to find elsewhere. Pc=Pc0NcPg=Pg0Ng
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


