Question: Swer oxidation is a useful alternative to using chromium based oxidizing agents, which are toxic. To begin Swer oxidation, dimethylsulfoxide (DMSO) is placed into oxalyl
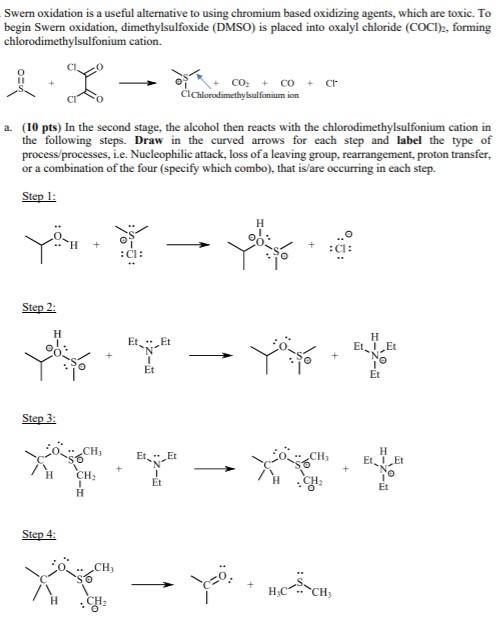
Swer oxidation is a useful alternative to using chromium based oxidizing agents, which are toxic. To begin Swer oxidation, dimethylsulfoxide (DMSO) is placed into oxalyl chloride (COCI), forming chlorodimethylsulfonium cation. CO; + co ClChlorodimethylsulfonium ion a. (10 pts) In the second stage, the alcohol then reacts with the chlorodimethylsuffonium cation in the following steps. Draw in the curved arrows for each step and label the type of process processes, le. Nucleophilic attack, loss of a leaving group. rearrangement, proton transfer, or a combination of the four (specify which combo), that is/are occurring in each step. Step 1: H H : CI: Step 2: H Et . Et H ELE Et Et Step 3: CH, CH, H ELE o Et CH: Et H Step 4: CH, Y+ HOT CH 1 CH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


