Question: T TH TH B QH cycle W! T D Q! 1 TL 1 D' S (a) (b) Figure 4-5: (a) Carnot cycle on the TS
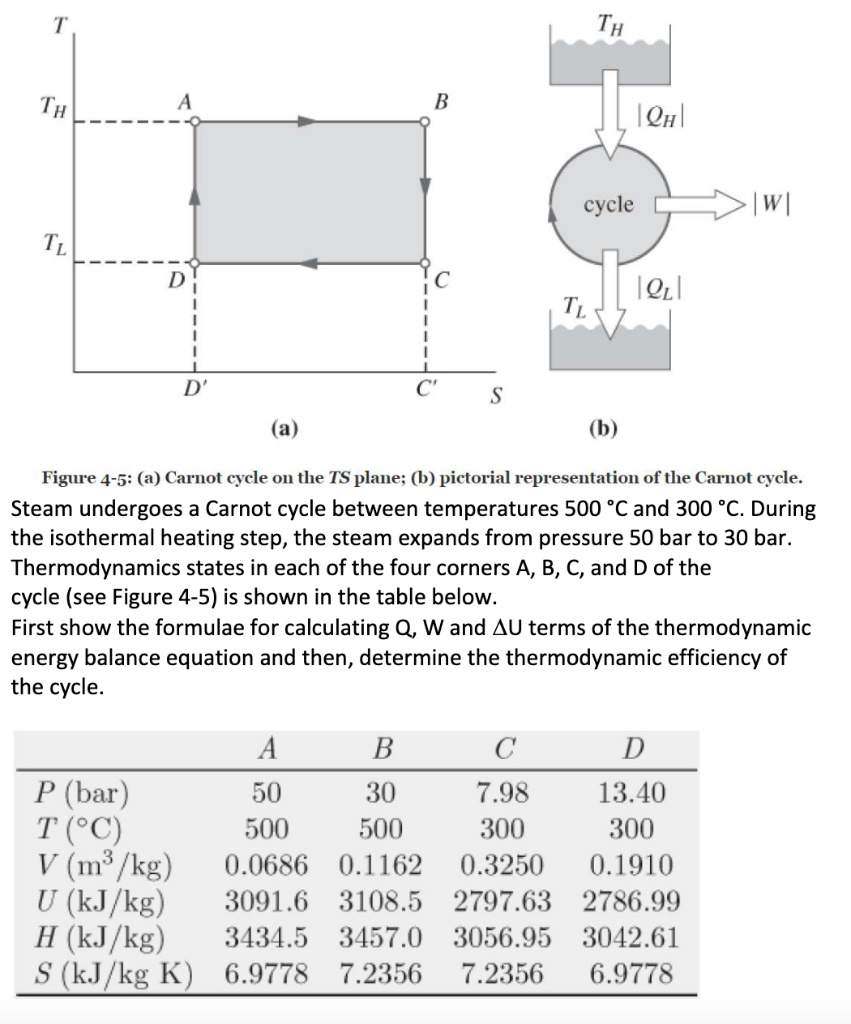
T TH TH B QH cycle W! T D Q! 1 TL 1 D' S (a) (b) Figure 4-5: (a) Carnot cycle on the TS plane; (b) pictorial representation of the Carnot cycle. Steam undergoes a Carnot cycle between temperatures 500 C and 300 C. During the isothermal heating step, the steam expands from pressure 50 bar to 30 bar. Thermodynamics states in each of the four corners A, B, C, and D of the cycle (see Figure 4-5) is shown in the table below. First show the formulae for calculating Q, W and AU terms of the thermodynamic energy balance equation and then, determine the thermodynamic efficiency of the cycle. B D P (bar) 50 30 7.98 13.40 T (C) 500 500 300 300 V (m3/kg) 0.0686 0.1162 0.3250 0.1910 U (kJ/kg) 3091.6 3108.5 2797.63 2786.99 H (kJ/kg) 3434.5 3457.0 3056.95 3042.61 S (kJ/kg K) 6.9778 7.2356 7.2356 6.9778
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


