Question: Table 1: Components of Solutions (Target Values) that will be used in this Spectrophotometer Experiment Flask Number/Type 1./BLANK 2./STANDARD 3./STANDARD 4./STANDARD 5. /STANDARD 6./UNKNOWN mL
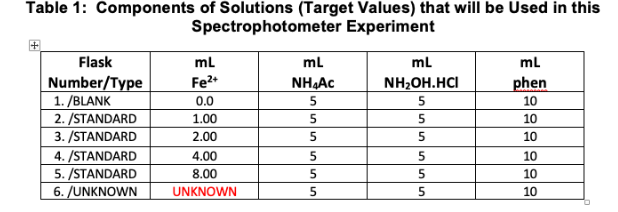

Table 1: Components of Solutions (Target Values) that will be used in this Spectrophotometer Experiment Flask Number/Type 1./BLANK 2./STANDARD 3./STANDARD 4./STANDARD 5. /STANDARD 6./UNKNOWN mL phen 10 10 mL Fe2+ 0.0 1.00 2.00 4.00 8.00 UNKNOWN ml NHAC 5 5 5 5 5 5 mL NH2OH.HCI 5 5 5 10 nunununun 5 5 5 10 10 10 Referring to Table 1 on page 9, what is the final concentration of Fe+2 in ppm of the last standard solution assuming that you used exactly the target value of 8.00 ml Fe+2 in the 100 ml flask? For this question, assume that you added exactly the volumes of all reagents as listed in the table. (Remember that the stock solution concentration is 50.00 ppm.) Show all your work carefully using good form and significant figures. Note that you will find the information on p. 16 to be useful here. [SF = 2, FORM = 3, ANS = 1]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


