Question: Table 2. Results. begin{tabular}{|l|c|c|c|} hline multicolumn{1}{|c|}{ Measured Quantity } & Trial 1 & Trial 2 & Trial 3 (optional) hline Mass of Salt (
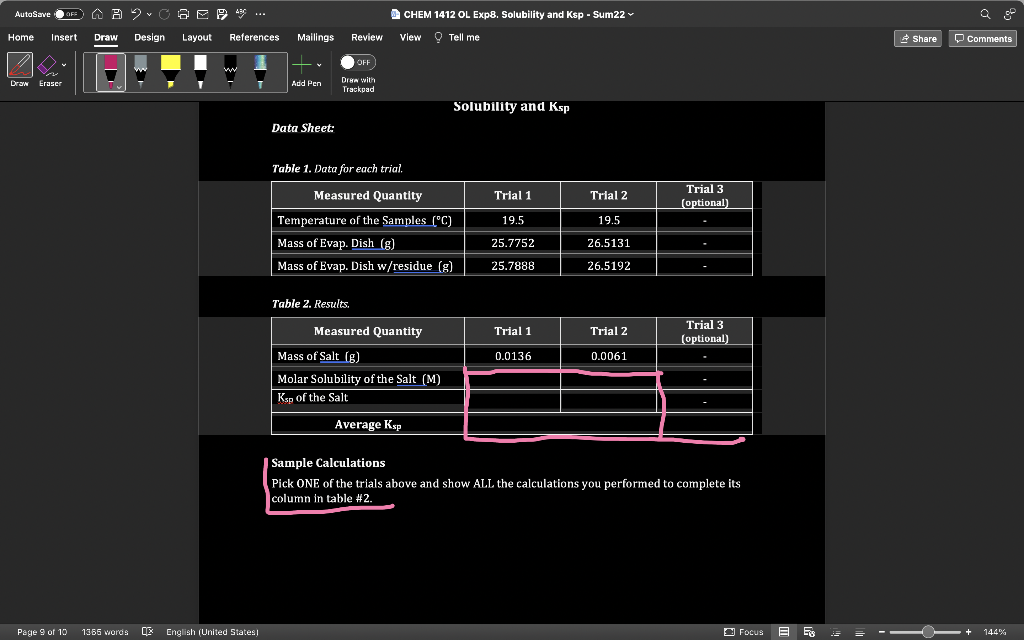
Table 2. Results. \begin{tabular}{|l|c|c|c|} \hline \multicolumn{1}{|c|}{ Measured Quantity } & Trial 1 & Trial 2 & Trial 3 (optional) \\ \hline Mass of Salt ( g ) & 0.0136 & 0.0061 & \\ \hline Molar Solubility of the Salt (M) & & & \\ \hline Ksp of the Salt & & & \\ \hline \multicolumn{1}{|c|}{ Average Ksp} & & \\ \hline \end{tabular} Sample Calculations Pick ONE of the trials above and show ALL the calculations you performed to complete its column in table #2. Table 2. Results. \begin{tabular}{|l|c|c|c|} \hline \multicolumn{1}{|c|}{ Measured Quantity } & Trial 1 & Trial 2 & Trial 3 (optional) \\ \hline Mass of Salt ( g ) & 0.0136 & 0.0061 & \\ \hline Molar Solubility of the Salt (M) & & & \\ \hline Ksp of the Salt & & & \\ \hline \multicolumn{1}{|c|}{ Average Ksp} & & \\ \hline \end{tabular} Sample Calculations Pick ONE of the trials above and show ALL the calculations you performed to complete its column in table #2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


