Question: Table view List view Titration of Unknown K,C,04 Sample Run 1 Run 2 Run 3 1.009 1.002 1.003 30.55 24.29 28 20 335 3.05 2.30
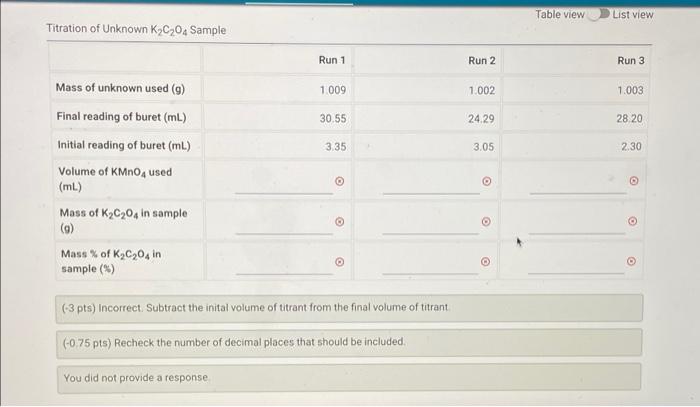
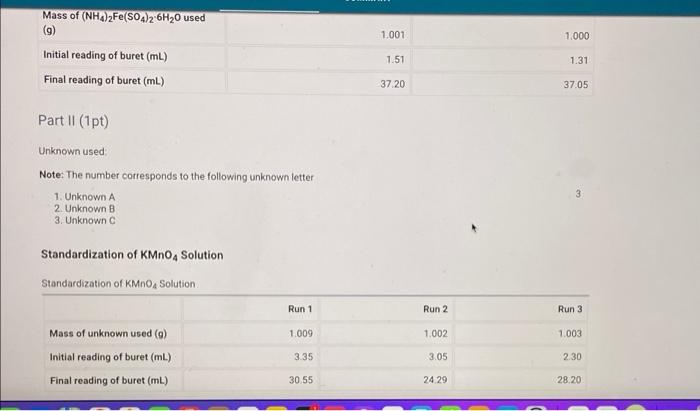
Table view List view Titration of Unknown K,C,04 Sample Run 1 Run 2 Run 3 1.009 1.002 1.003 30.55 24.29 28 20 335 3.05 2.30 Mass of unknown used (9) Final reading of buret (mL) Initial reading of buret (ml.) Volume of KMnO4 used (mL) Moss of K C204 in sample (9) Mass % of K C204 in sample (%) O 0 (-3 pts) Incorrect. Subtract the inital volume of titrant from the final volume of titrant (-0.75 pts) Recheck the number of decimal places that should be included. You did not provide a response 1.001 1.000 Mass of (NH4)2Fe(504)2-6H20 used 9) Initial reading of buret (mL) Final reading of buret (mL) 1.51 1.31 37.20 37.05 Part II (1 pt) Unknown used Note: The number corresponds to the following unknown letter 1. Unknown A 2 Unknown 3. Unknown 3 Standardization of KMnO4 Solution Standardization of KMnO4 Solution Run 1 Run 2 Run 3 1.009 1.002 1.003 Mass of unknown used (m) Initial reading of buret (ml) 3.35 3.05 2.30 Final reading of buret (mL) 30.55 24.29 28.20
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


