Question: Tables: Could somebody help me to solve this problem with a step-by-step process explanation? It doesn't have to be a super detailed one since I

Tables:
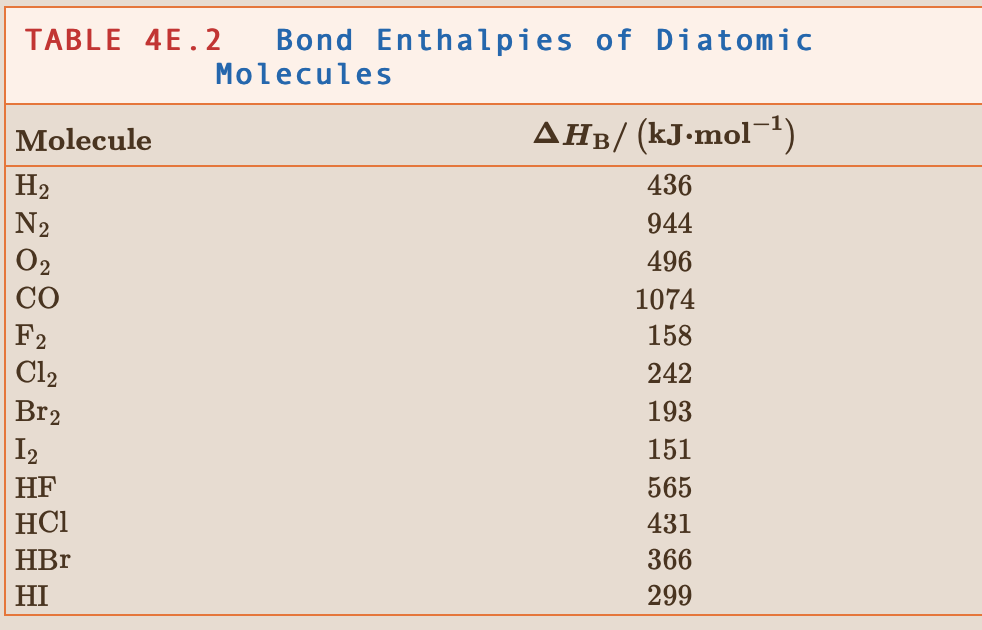
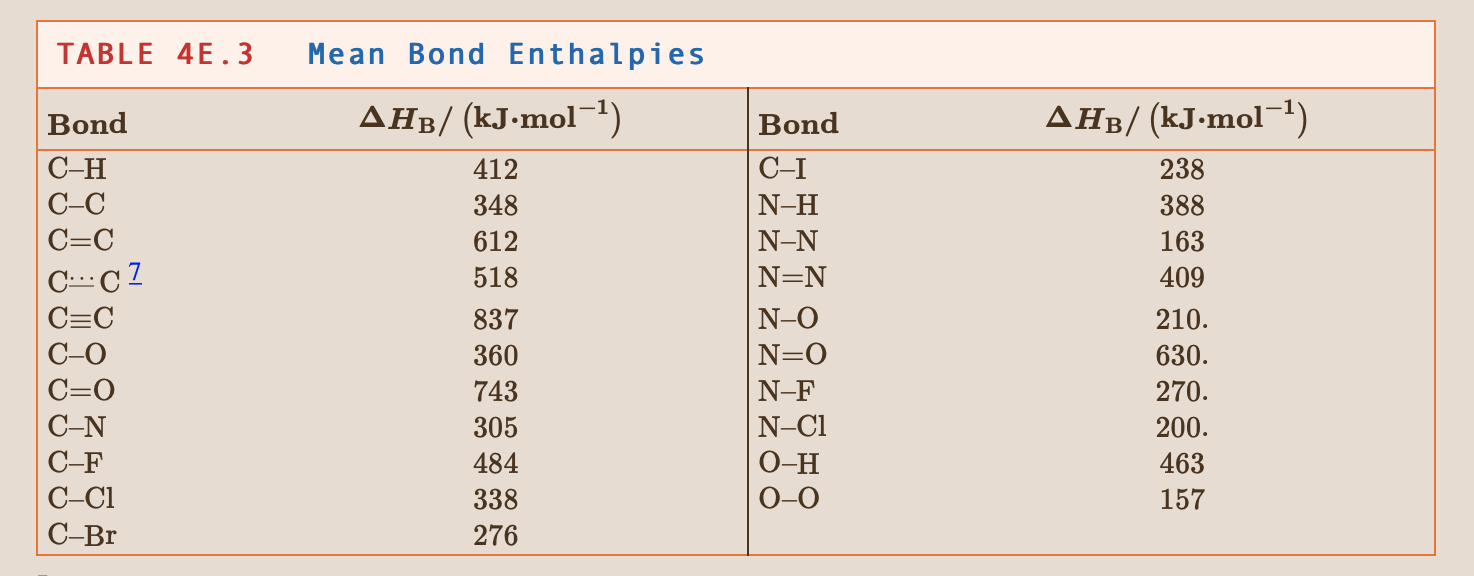
Could somebody help me to solve this problem with a step-by-step process explanation? It doesn't have to be a super detailed one since I do believe that I have the right conceptual comprehension of reaction enthalpy, but not sure how to solve one for some reason :/
thank you so much!
4.5 Use the bond enthalpies in Tables 48.2 and 48.3 to estimate the reaction enthalpy for (a) 3C2H2(g) C6H6(g) TABLE 45.2 Bond Enthalpies of Diatomic Molecules Molecule H2 |N2 02 CO F2 Cl2 Br2 AHB/ (kJ.mol-1) 436 944 496 1074 158 242 193 151 565 431 366 299 12 HF HBr HI TABLE 48.3 Bond C-H C-C C=C C.-.CZ C=C C-O C=0 C-N C-F C-C1 C-Br Mean Bond Enthalpies AHp/ (kJ.mol-1) 412 348 612 518 837 360 743 305 484 338 276 Bond C-I N-H N-N NON N-O N=0 N-F N-CI 0-H 0-0 AHB/(kJ.mol-1) 238 388 163 409 210. 630. 270. 200. 463 157
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


