Question: Task 2 Q2. A gas mixture containing A mole % CO2 and B mole % ethanol is passed through an absorption tower to separate ethanol
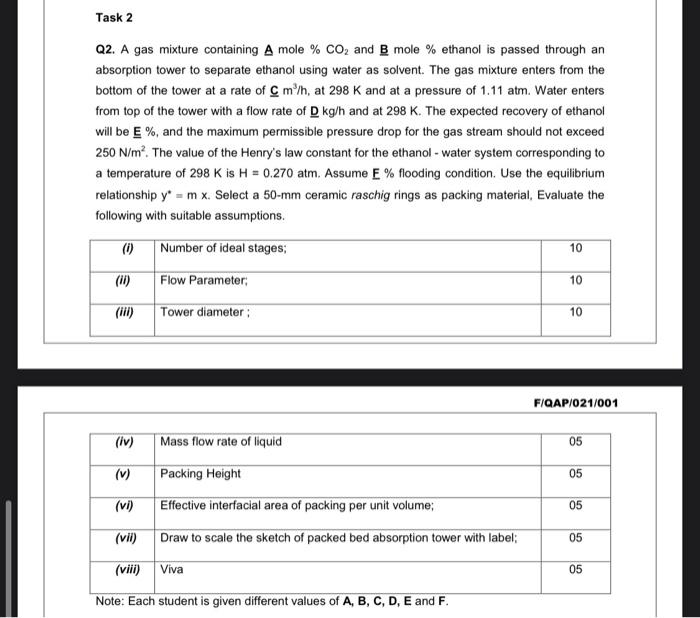
Task 2 Q2. A gas mixture containing A mole % CO2 and B mole % ethanol is passed through an absorption tower to separate ethanol using water as solvent. The gas mixture enters from the bottom of the tower at a rate of C m/h, at 298 K and at a pressure of 1.11 atm. Water enters from top of the tower with a flow rate of D kg/h and at 298 K. The expected recovery of ethanol will be E %, and the maximum permissible pressure drop for the gas stream should not exceed 250 N/m. The value of the Henry's law constant for the ethanol - water system corresponding to a temperature of 298 K is H = 0.270 atm. Assume E % flooding condition. Use the equilibrium relationship y = m x. Select a 50-mm ceramic raschig rings as packing material, Evaluate the following with suitable assumptions. (i) Number of ideal stages; 10 (11) Flow Parameter 10 Tower diameter: 10 FIQAP/021/001 (iv) Mass flow rate of liquid 05 (v) 05 Packing Height Effective interfacial area of packing per unit volume; (vi) 05 (vii) Draw to scale the sketch of packed bed absorption tower with label: 05 (viii) Viva 05 Note: Each student is given different values of A, B, C, D, E and F
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


