Question: Task 3 part B A compressed air storage cylinder has a volume of OS m3 and contains air at an absolute pressure of 1.8 MPa
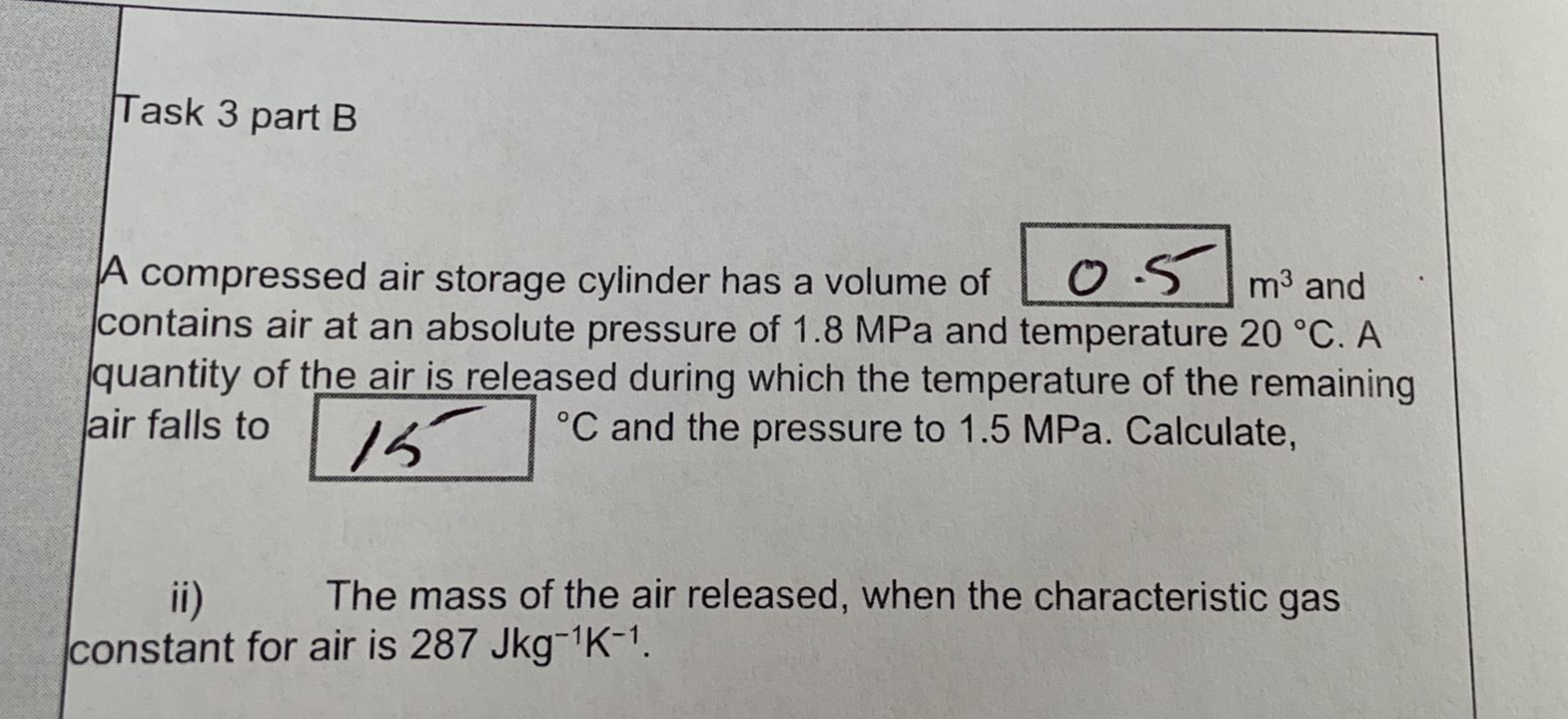
Task 3 part B A compressed air storage cylinder has a volume of OS m3 and contains air at an absolute pressure of 1.8 MPa and temperature 20 .C. A quantity of the air is released during which the temperature of the remaining air falls to 15 .C and the pressure to 1.5 MPa. Calculate, ii) The mass of the air released, when the characteristic gas constant for air is 287 Jkg-1K-1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


