Question: Task 3 : Refrigerator with nitrogen There is an open insulated bottle containing liquid nitrogen in a refrigerator. The refrigerator is neither perfectly insulated nor
Task : Refrigerator with nitrogen
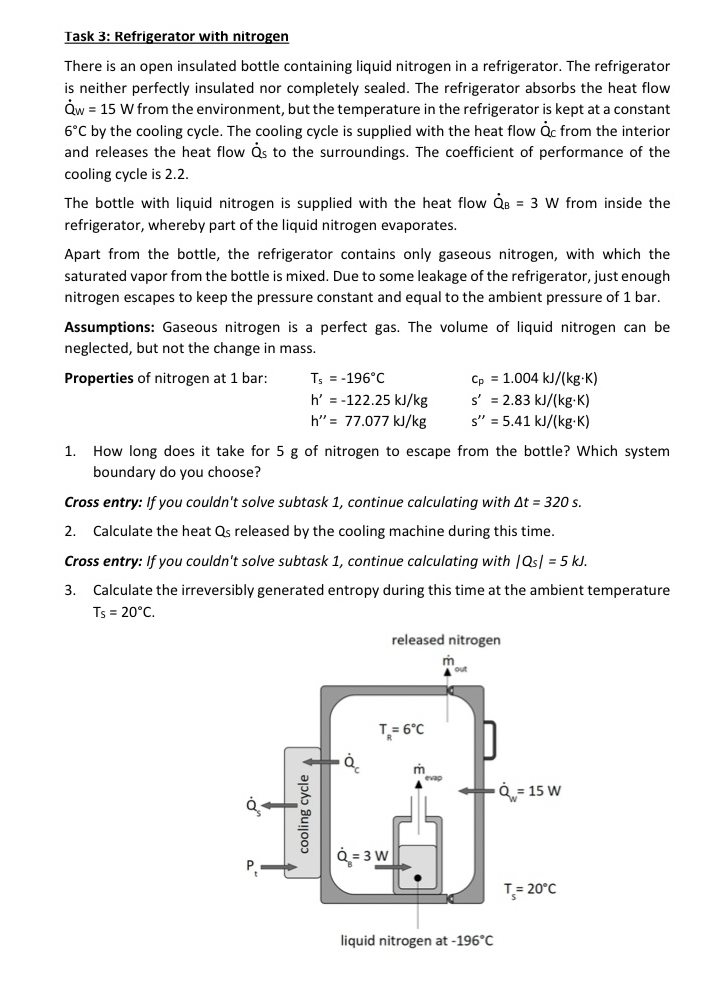
There is an open insulated bottle containing liquid nitrogen in a refrigerator. The refrigerator is neither perfectly insulated nor completely sealed. The refrigerator absorbs the heat flow from the environment, but the temperature in the refrigerator is kept at a constant by the cooling cycle. The cooling cycle is supplied with the heat flow from the interior and releases the heat flow to the surroundings. The coefficient of performance of the cooling cycle is
The bottle with liquid nitrogen is supplied with the heat flow from inside the refrigerator, whereby part of the liquid nitrogen evaporates.
Apart from the bottle, the refrigerator contains only gaseous nitrogen, with which the saturated vapor from the bottle is mixed. Due to some leakage of the refrigerator, just enough nitrogen escapes to keep the pressure constant and equal to the ambient pressure of bar.
Assumptions: Gaseous nitrogen is a perfect gas. The volume of liquid nitrogen can be neglected, but not the change in mass.
Properties of nitrogen at bar:
How long does it take for g of nitrogen to escape from the bottle? Which system boundary do you choose?
Cross entry: If you couldn't solve subtask continue calculating with
Calculate the heat released by the cooling machine during this time.
Cross entry: If you couldn't solve subtask continue calculating with
Calculate the irreversibly generated entropy during this time at the ambient temperature
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


