Question: Temperature (C) 105 100 95 90 85 80 75 70 65 Methanol-Water vapor-liquid equilibrium data P = 1 atm 60 0 0.1 0.2 0.3
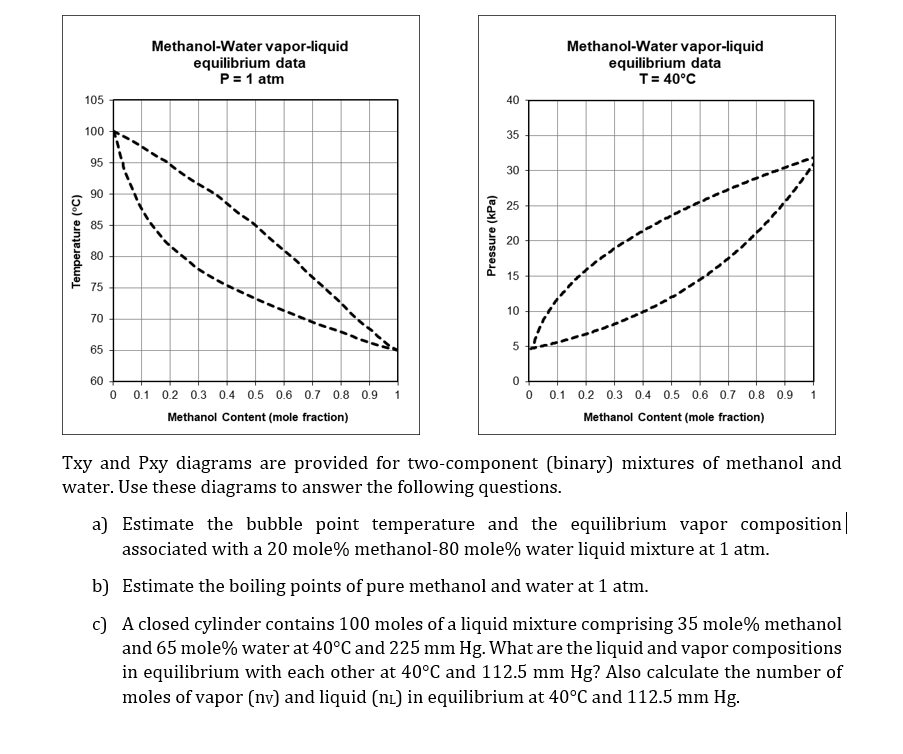
Temperature (C) 105 100 95 90 85 80 75 70 65 Methanol-Water vapor-liquid equilibrium data P = 1 atm 60 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 Methanol Content (mole fraction) Pressure (kPa) 40 35 30 25 20 15 10 5 Methanol-Water vapor-liquid equilibrium data T = 40C 0 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 Methanol Content (mole fraction) Txy and Pxy diagrams are provided for two-component (binary) mixtures water. Use these diagrams to answer the following questions. a) Estimate the bubble point temperature and the equilibrium vapor composition | associated with a 20 mole% methanol-80 mole% water liquid mixture at 1 atm. b) Estimate the boiling points of pure methanol and water at 1 atm. c) A closed cylinder contains 100 moles of a liquid mixture comprising 35 mole% methanol and 65 mole% water at 40C and 225 mm Hg. What are the liquid and vapor compositions in equilibrium with each other at 40C and 112.5 mm Hg? Also calculate the number of moles of vapor (nv) and liquid (n) in equilibrium at 40C and 112.5 mm Hg.
Step by Step Solution
3.36 Rating (146 Votes )
There are 3 Steps involved in it
This has to calculated from the figure Ill help you with the steps and answer a P 1 atm methanol is 20 ie x02 We have to find Bubble point temperature ... View full answer

Get step-by-step solutions from verified subject matter experts


