Question: Temperature Temperature 3000 y = Austenite Q = Ferrite 8 - Delta iron CM = Cementite 8 +L 2883 2720 1539 1492 2600 2552 CM
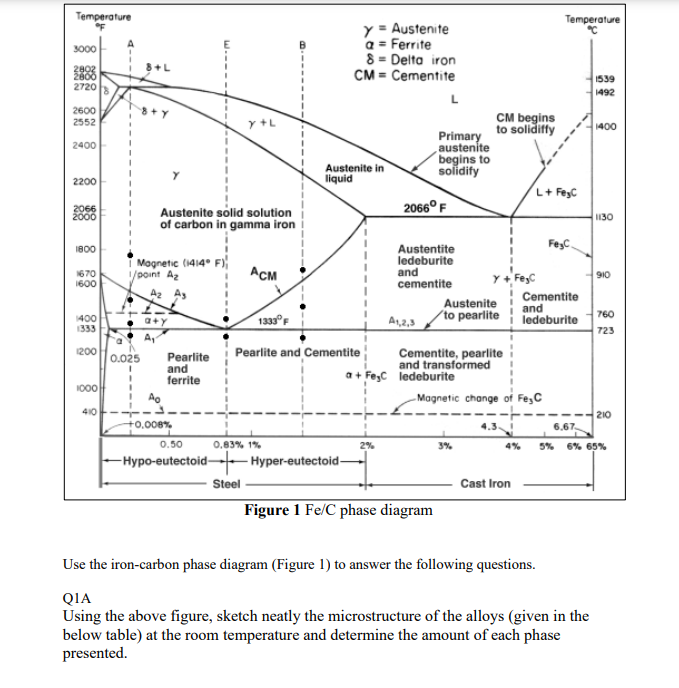
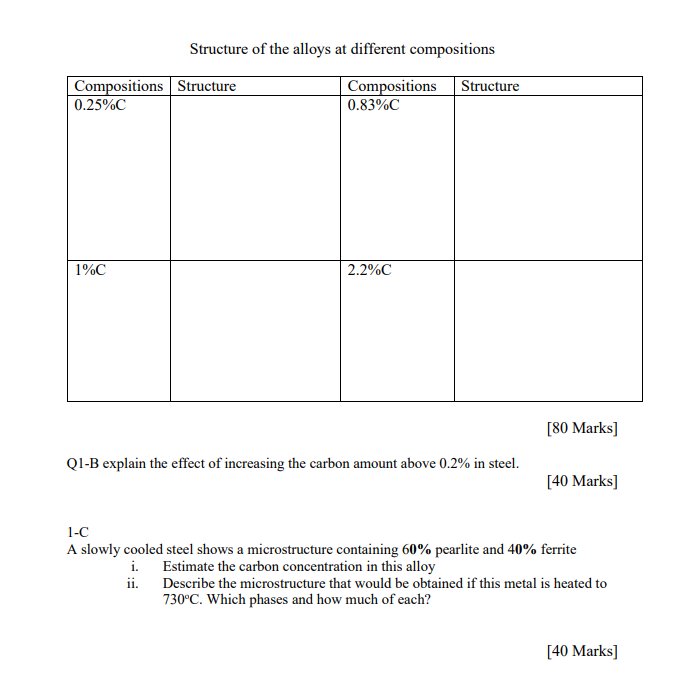
Temperature Temperature 3000 y = Austenite Q = Ferrite 8 - Delta iron CM = Cementite 8 +L 2883 2720 1539 1492 2600 2552 CM begins to solidiffy 1400 2400 Primary austente begins to solidify Austenite in liquid 2200 L+ Feyc 2066 2000 2066F Austenite solid solution of carbon in gamma iron 1130 1800 Feyc 1670 1600 Magnetic (1414 F) 1/point Az Az As SIO Austentite ledeburite and cementite Austenite A1,2,3 to pearlite Cementite and ledeburite 760 1400 1333 a+Y 1333 723 1200 0.025 1 1 1 ! A Pearlite and ferrite Pearlite and Cementite Cementite, pearlite and transformed a + Fec ledeburite Magnetic change of Fejc 1000 410 210 +0.008% 4.3 6.67 0.50 0.83% 1% 2% 3%. 4% 5% 6% 65% -Hypo-eutectoid-5 Hyper-eutectoid- Steel Figure 1 Fe/C phase diagram Cast Iron Use the iron-carbon phase diagram (Figure 1) to answer the following questions. QIA Using the above figure, sketch neatly the microstructure of the alloys (given in the below table) at the room temperature and determine the amount of each phase presented. Structure of the alloys at different compositions Compositions Structure 0.25%C Compositions Structure 0.83%C 1%C 2.2%C [80 Marks] Q1-B explain the effect of increasing the carbon amount above 0.2% in steel. [40 Marks] 1-C A slowly cooled steel shows a microstructure containing 60% pearlite and 40% ferrite i. Estimate the carbon concentration in this alloy ii. Describe the microstructure that would be obtained if this metal is heated to 730C. Which phases and how much of each? [40 Marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


