Question: Test with water at room temperature: Explain why when mixing these metals from the table below with water at room temperature these changes occur:
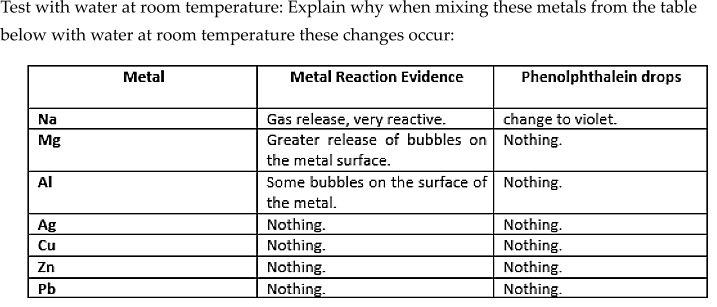
Test with water at room temperature: Explain why when mixing these metals from the table below with water at room temperature these changes occur: Metal Metal Reaction Evidence Phenolphthalein drops Gas release, very reactive. Greater release of bubbles on Nothing. change to violet. Na Mg the metal surface. Al Some bubbles on the surface of | Nothing. the metal. Nothing. Nothing. Nothing. Nothing. Nothing. Ag Cu Zn Nothing. Pb Nothing. Nothing.
Step by Step Solution
3.50 Rating (153 Votes )
There are 3 Steps involved in it
Phenolphthalein indicator turns deep orange in strongly acidic solutions will turn colorless in lightly acidic or near neutral solutions It will be pi... View full answer

Get step-by-step solutions from verified subject matter experts


