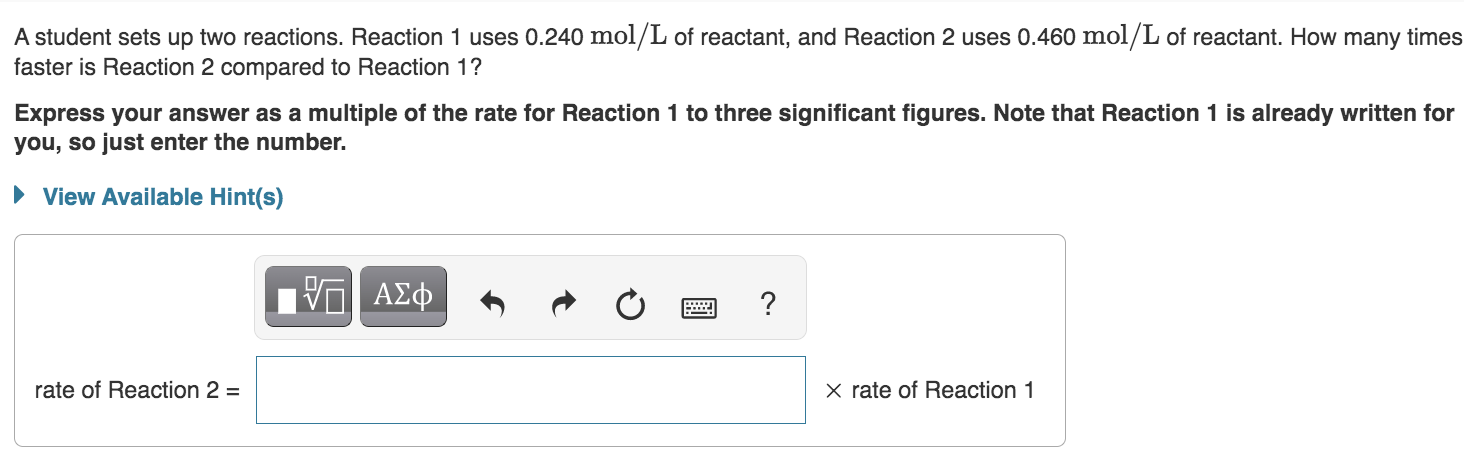
Question: Thank you for any help understanding this concept A student sets up two reactions. Reaction 1 uses 0.240mol/L of reactant, and Reaction 2 uses 0.460mol/L
 Thank you for any help understanding this concept
Thank you for any help understanding this concept
A student sets up two reactions. Reaction 1 uses 0.240mol/L of reactant, and Reaction 2 uses 0.460mol/L of reactant. How many times faster is Reaction 2 compared to Reaction 1 ? Express your answer as a multiple of the rate for Reaction 1 to three significant figures. Note that Reaction 1 is already written for you, so just enter the number
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


